| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Neurotensin receptor type 2 |
|---|
| Ligand | BDBM50322368 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_643026 (CHEMBL1176540) |
|---|
| Kd | 23±n/a nM |
|---|
| Citation |  Hughes, FM; Shaner, BE; May, LA; Zotian, L; Brower, JO; Woods, RJ; Cash, M; Morrow, D; Massa, F; Mazella, J; Dix, TA Identification and functional characterization of a stable, centrally active derivative of the neurotensin (8-13) fragment as a potential first-in-class analgesic. J Med Chem53:4623-32 (2010) [PubMed] Article Hughes, FM; Shaner, BE; May, LA; Zotian, L; Brower, JO; Woods, RJ; Cash, M; Morrow, D; Massa, F; Mazella, J; Dix, TA Identification and functional characterization of a stable, centrally active derivative of the neurotensin (8-13) fragment as a potential first-in-class analgesic. J Med Chem53:4623-32 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Neurotensin receptor type 2 |
|---|
| Name: | Neurotensin receptor type 2 |
|---|
| Synonyms: | High-affinity levocabastine-sensitive neurotensin receptor | NT-R-2 | NTR2_RAT | Neurotensin receptor 2 | Neurotensin receptor type 2 | Ntr2 | Ntsr2 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 46280.50 |
|---|
| Organism: | Rattus norvegicus |
|---|
| Description: | ChEMBL_1466615 |
|---|
| Residue: | 416 |
|---|
| Sequence: | METSSPWPPRPSPSAGLSLEARLGVDTRLWAKVLFTALYSLIFAFGTAGNALSVHVVLKA
RAGRPGRLRYHVLSLALSALLLLLVSMPMELYNFVWSHYPWVFGDLGCRGYYFVRELCAY
ATVLSVASLSAERCLAVCQPLRARRLLTPRRTRRLLSLVWVASLGLALPMAVIMGQKHEV
ESADGEPEPASRVCTVLVSRATLQVFIQVNVLVSFALPLALTAFLNGITVNHLMALYSQV
PSASAQVSSIPSRLELLSEEGLLGFITWRKTLSLGVQASLVRHKDASQIRSLQHSAQVLR
AIVAVYVICWLPYHARRLMYCYIPDDGWTNELYDFYHYFYMVTNTLFYVSSAVTPILYNA
VSSSFRKLFLESLGSLCGEQHSLVPLPQEAPESTTSTYSFRLWGSPRNPSLGEIQV
|
|
|
|---|
| BDBM50322368 |
|---|
| n/a |
|---|
| Name | BDBM50322368 |
|---|
| Synonyms: | (S)-5-((S)-1-((S)-2-((S)-1-((S)-1-((S)-1-carboxy-3-methylbutylamino)-3,3-dimethyl-1-oxobutan-2-ylamino)-3-(4-hydroxyphenyl)-1-oxopropan-2-ylcarbamoyl)pyrrolidin-1-yl)-5-guanidino-1-oxopentan-2-ylamino)-N,N,N,4-tetramethyl-5-oxopentan-1-aminium | CHEMBL1172376 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C41H70N9O8 |
|---|
| Mol. Mass. | 817.0495 |
|---|
| SMILES | CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)CCC[N+](C)(C)C)C(C)(C)C)C(O)=O |r| |
|---|
| Structure | 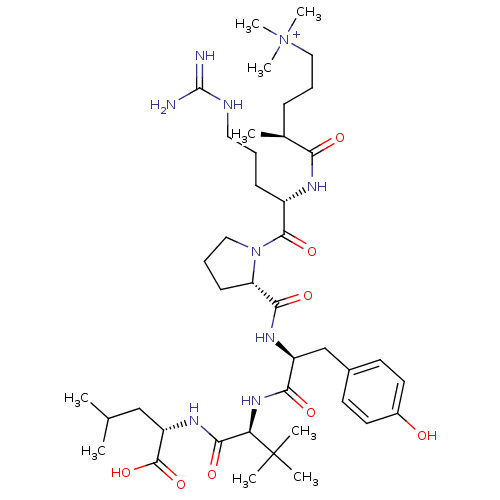
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Hughes, FM; Shaner, BE; May, LA; Zotian, L; Brower, JO; Woods, RJ; Cash, M; Morrow, D; Massa, F; Mazella, J; Dix, TA Identification and functional characterization of a stable, centrally active derivative of the neurotensin (8-13) fragment as a potential first-in-class analgesic. J Med Chem53:4623-32 (2010) [PubMed] Article
Hughes, FM; Shaner, BE; May, LA; Zotian, L; Brower, JO; Woods, RJ; Cash, M; Morrow, D; Massa, F; Mazella, J; Dix, TA Identification and functional characterization of a stable, centrally active derivative of the neurotensin (8-13) fragment as a potential first-in-class analgesic. J Med Chem53:4623-32 (2010) [PubMed] Article