| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Nitric oxide synthase, brain |
|---|
| Ligand | BDBM50325184 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_651797 (CHEMBL1225413) |
|---|
| Ki | 4300±n/a nM |
|---|
| Citation |  Xue, F; Huang, J; Ji, H; Fang, J; Li, H; Martásek, P; Roman, LJ; Poulos, TL; Silverman, RB Structure-based design, synthesis, and biological evaluation of lipophilic-tailed monocationic inhibitors of neuronal nitric oxide synthase. Bioorg Med Chem18:6526-37 (2010) [PubMed] Article Xue, F; Huang, J; Ji, H; Fang, J; Li, H; Martásek, P; Roman, LJ; Poulos, TL; Silverman, RB Structure-based design, synthesis, and biological evaluation of lipophilic-tailed monocationic inhibitors of neuronal nitric oxide synthase. Bioorg Med Chem18:6526-37 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Nitric oxide synthase, brain |
|---|
| Name: | Nitric oxide synthase, brain |
|---|
| Synonyms: | Bnos | N-NOS | NC-NOS | NOS | NOS type I nNOS | NOS1_RAT | Neuronal nitric oxide synthase | Neuronal nitric oxide synthase (nNOS) | Nitric Oxide Synthase, brain | Nitric oxide synthase (nNOS) | Nitric oxide synthase, brain (nNOS) | Nitric-oxide synthase, brain | Nitric-oxide synthase, brain (nNOS) | Nitrogen oxide synthase - neuronal | Nos1 | Peptidyl-cysteine S-nitrosylase NOS1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 160570.98 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Recombinant nNOS overexpressed in E. coli was used in enzyme assays. |
|---|
| Residue: | 1429 |
|---|
| Sequence: | MEENTFGVQQIQPNVISVRLFKRKVGGLGFLVKERVSKPPVIISDLIRGGAAEQSGLIQA
GDIILAVNDRPLVDLSYDSALEVLRGIASETHVVLILRGPEGFTTHLETTFTGDGTPKTI
RVTQPLGPPTKAVDLSHQPSASKDQSLAVDRVTGLGNGPQHAQGHGQGAGSVSQANGVAI
DPTMKSTKANLQDIGEHDELLKEIEPVLSILNSGSKATNRGGPAKAEMKDTGIQVDRDLD
GKSHKAPPLGGDNDRVFNDLWGKDNVPVILNNPYSEKEQSPTSGKQSPTKNGSPSRCPRF
LKVKNWETDVVLTDTLHLKSTLETGCTEHICMGSIMLPSQHTRKPEDVRTKDQLFPLAKE
FLDQYYSSIKRFGSKAHMDRLEEVNKEIESTSTYQLKDTELIYGAKHAWRNASRCVGRIQ
WSKLQVFDARDCTTAHGMFNYICNHVKYATNKGNLRSAITIFPQRTDGKHDFRVWNSQLI
RYAGYKQPDGSTLGDPANVQFTEICIQQGWKAPRGRFDVLPLLLQANGNDPELFQIPPEL
VLEVPIRHPKFDWFKDLGLKWYGLPAVSNMLLEIGGLEFSACPFSGWYMGTEIGVRDYCD
NSRYNILEEVAKKMDLDMRKTSSLWKDQALVEINIAVLYSFQSDKVTIVDHHSATESFIK
HMENEYRCRGGCPADWVWIVPPMSGSITPVFHQEMLNYRLTPSFEYQPDPWNTHVWKGTN
GTPTKRRAIGFKKLAEAVKFSAKLMGQAMAKRVKATILYATETGKSQAYAKTLCEIFKHA
FDAKAMSMEEYDIVHLEHEALVLVVTSTFGNGDPPENGEKFGCALMEMRHPNSVQEERKS
YKVRFNSVSSYSDSRKSSGDGPDLRDNFESTGPLANVRFSVFGLGSRAYPHFCAFGHAVD
TLLEELGGERILKMREGDELCGQEEAFRTWAKKVFKAACDVFCVGDDVNIEKPNNSLISN
DRSWKRNKFRLTYVAEAPDLTQGLSNVHKKRVSAARLLSRQNLQSPKFSRSTIFVRLHTN
GNQELQYQPGDHLGVFPGNHEDLVNALIERLEDAPPANHVVKVEMLEERNTALGVISNWK
DESRLPPCTIFQAFKYYLDITTPPTPLQLQQFASLATNEKEKQRLLVLSKGLQEYEEWKW
GKNPTMVEVLEEFPSIQMPATLLLTQLSLLQPRYYSISSSPDMYPDEVHLTVAIVSYHTR
DGEGPVHHGVCSSWLNRIQADDVVPCFVRGAPSFHLPRNPQVPCILVGPGTGIAPFRSFW
QQRQFDIQHKGMNPCPMVLVFGCRQSKIDHIYREETLQAKNKGVFRELYTAYSREPDRPK
KYVQDVLQEQLAESVYRALKEQGGHIYVCGDVTMAADVLKAIQRIMTQQGKLSEEDAGVF
ISRLRDDNRYHEDIFGVTLRTYEVTNRLRSESIAFIEESKKDADEVFSS
|
|
|
|---|
| BDBM50325184 |
|---|
| n/a |
|---|
| Name | BDBM50325184 |
|---|
| Synonyms: | 6-(((3R,4R/3S,4S)-4-(3-(4-Fluoro-3-methylphenoxy)-phenoxy)pyrrolidin-3-yl)methyl)pyridin-2-amine | CHEMBL1222676 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H24FN3O2 |
|---|
| Mol. Mass. | 393.454 |
|---|
| SMILES | Cc1cc(Oc2cccc(O[C@H]3CNC[C@H]3Cc3cccc(N)n3)c2)ccc1F |r| |
|---|
| Structure | 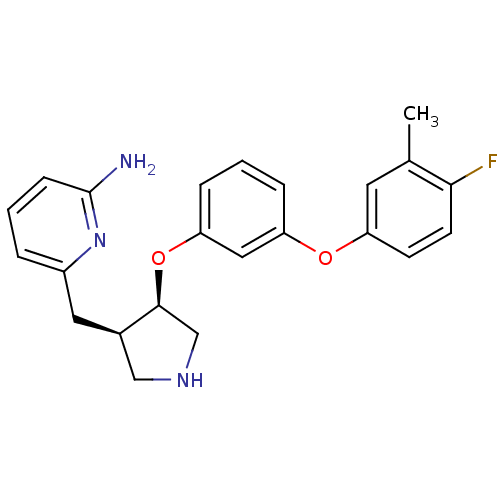
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Xue, F; Huang, J; Ji, H; Fang, J; Li, H; Martásek, P; Roman, LJ; Poulos, TL; Silverman, RB Structure-based design, synthesis, and biological evaluation of lipophilic-tailed monocationic inhibitors of neuronal nitric oxide synthase. Bioorg Med Chem18:6526-37 (2010) [PubMed] Article
Xue, F; Huang, J; Ji, H; Fang, J; Li, H; Martásek, P; Roman, LJ; Poulos, TL; Silverman, RB Structure-based design, synthesis, and biological evaluation of lipophilic-tailed monocationic inhibitors of neuronal nitric oxide synthase. Bioorg Med Chem18:6526-37 (2010) [PubMed] Article