| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histone acetyltransferase p300 |
|---|
| Ligand | BDBM50328743 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_675529 (CHEMBL1273742) |
|---|
| IC50 | 13700±n/a nM |
|---|
| Citation |  Feng, Y; Li, M; Wang, B; Zheng, YG Discovery and mechanistic study of a class of protein arginine methylation inhibitors. J Med Chem53:6028-39 (2010) [PubMed] Article Feng, Y; Li, M; Wang, B; Zheng, YG Discovery and mechanistic study of a class of protein arginine methylation inhibitors. J Med Chem53:6028-39 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histone acetyltransferase p300 |
|---|
| Name: | Histone acetyltransferase p300 |
|---|
| Synonyms: | EP300 | EP300_HUMAN | HIF1A/p300/CREB-binding protein | Histone acetyltransferase p300/Hypoxia-inducible factor 1-alpha | P300 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 264223.04 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q09472 |
|---|
| Residue: | 2414 |
|---|
| Sequence: | MAENVVEPGPPSAKRPKLSSPALSASASDGTDFGSLFDLEHDLPDELINSTELGLTNGGD
INQLQTSLGMVQDAASKHKQLSELLRSGSSPNLNMGVGGPGQVMASQAQQSSPGLGLINS
MVKSPMTQAGLTSPNMGMGTSGPNQGPTQSTGMMNSPVNQPAMGMNTGMNAGMNPGMLAA
GNGQGIMPNQVMNGSIGAGRGRQNMQYPNPGMGSAGNLLTEPLQQGSPQMGGQTGLRGPQ
PLKMGMMNNPNPYGSPYTQNPGQQIGASGLGLQIQTKTVLSNNLSPFAMDKKAVPGGGMP
NMGQQPAPQVQQPGLVTPVAQGMGSGAHTADPEKRKLIQQQLVLLLHAHKCQRREQANGE
VRQCNLPHCRTMKNVLNHMTHCQSGKSCQVAHCASSRQIISHWKNCTRHDCPVCLPLKNA
GDKRNQQPILTGAPVGLGNPSSLGVGQQSAPNLSTVSQIDPSSIERAYAALGLPYQVNQM
PTQPQVQAKNQQNQQPGQSPQGMRPMSNMSASPMGVNGGVGVQTPSLLSDSMLHSAINSQ
NPMMSENASVPSLGPMPTAAQPSTTGIRKQWHEDITQDLRNHLVHKLVQAIFPTPDPAAL
KDRRMENLVAYARKVEGDMYESANNRAEYYHLLAEKIYKIQKELEEKRRTRLQKQNMLPN
AAGMVPVSMNPGPNMGQPQPGMTSNGPLPDPSMIRGSVPNQMMPRITPQSGLNQFGQMSM
AQPPIVPRQTPPLQHHGQLAQPGALNPPMGYGPRMQQPSNQGQFLPQTQFPSQGMNVTNI
PLAPSSGQAPVSQAQMSSSSCPVNSPIMPPGSQGSHIHCPQLPQPALHQNSPSPVPSRTP
TPHHTPPSIGAQQPPATTIPAPVPTPPAMPPGPQSQALHPPPRQTPTPPTTQLPQQVQPS
LPAAPSADQPQQQPRSQQSTAASVPTPTAPLLPPQPATPLSQPAVSIEGQVSNPPSTSST
EVNSQAIAEKQPSQEVKMEAKMEVDQPEPADTQPEDISESKVEDCKMESTETEERSTELK
TEIKEEEDQPSTSATQSSPAPGQSKKKIFKPEELRQALMPTLEALYRQDPESLPFRQPVD
PQLLGIPDYFDIVKSPMDLSTIKRKLDTGQYQEPWQYVDDIWLMFNNAWLYNRKTSRVYK
YCSKLSEVFEQEIDPVMQSLGYCCGRKLEFSPQTLCCYGKQLCTIPRDATYYSYQNRYHF
CEKCFNEIQGESVSLGDDPSQPQTTINKEQFSKRKNDTLDPELFVECTECGRKMHQICVL
HHEIIWPAGFVCDGCLKKSARTRKENKFSAKRLPSTRLGTFLENRVNDFLRRQNHPESGE
VTVRVVHASDKTVEVKPGMKARFVDSGEMAESFPYRTKALFAFEEIDGVDLCFFGMHVQE
YGSDCPPPNQRRVYISYLDSVHFFRPKCLRTAVYHEILIGYLEYVKKLGYTTGHIWACPP
SEGDDYIFHCHPPDQKIPKPKRLQEWYKKMLDKAVSERIVHDYKDIFKQATEDRLTSAKE
LPYFEGDFWPNVLEESIKELEQEEEERKREENTSNESTDVTKGDSKNAKKKNNKKTSKNK
SSLSRGNKKKPGMPNVSNDLSQKLYATMEKHKEVFFVIRLIAGPAANSLPPIVDPDPLIP
CDLMDGRDAFLTLARDKHLEFSSLRRAQWSTMCMLVELHTQSQDRFVYTCNECKHHVETR
WHCTVCEDYDLCITCYNTKNHDHKMEKLGLGLDDESNNQQAAATQSPGDSRRLSIQRCIQ
SLVHACQCRNANCSLPSCQKMKRVVQHTKGCKRKTNGGCPICKQLIALCCYHAKHCQENK
CPVPFCLNIKQKLRQQQLQHRLQQAQMLRRRMASMQRTGVVGQQQGLPSPTPATPTTPTG
QQPTTPQTPQPTSQPQPTPPNSMPPYLPRTQAAGPVSQGKAAGQVTPPTPPQTAQPPLPG
PPPAAVEMAMQIQRAAETQRQMAHVQIFQRPIQHQMPPMTPMAPMGMNPPPMTRGPSGHL
EPGMGPTGMQQQPPWSQGGLPQPQQLQSGMPRPAMMSVAQHGQPLNMAPQPGLGQVGISP
LKPGTVSQQALQNLLRTLRSPSSPLQQQQVLSILHANPQLLAAFIKQRAAKYANSNPQPI
PGQPGMPQGQPGLQPPTMPGQQGVHSNPAMQNMNPMQAGVQRAGLPQQQPQQQLQPPMGG
MSPQAQQMNMNHNTMPSQFRDILRRQQMMQQQQQQGAGPGIGPGMANHNQFQQPQGVGYP
PQQQQRMQHHMQQMQQGNMGQIGQLPQALGAEAGASLQAYQQRLLQQQMGSPVQPNPMSP
QQHMLPNQAQSPHLQGQQIPNSLSNQVRSPQPVPSPRPQSQPPHSSPSPRMQPQPSPHHV
SPQTSSPHPGLVAAQANPMEQGHFASPDQNSMLSQLASNPGMANLHGASATDLGLSTDNS
DLNSNLSQSTLDIH
|
|
|
|---|
| BDBM50328743 |
|---|
| n/a |
|---|
| Name | BDBM50328743 |
|---|
| Synonyms: | 3-((4-acetamidophenyl)diazenyl)-4-hydroxy-7-(3-(5-hydroxy-6-((E)-phenyldiazenyl)-7-sulfonaphthalen-2-yl)ureido)naphthalene-2-sulfonate | 3-((4-acetamidophenyl)diazenyl)-4-hydroxy-7-(3-(5-hydroxy-6-(phenyldiazenyl)-7-sulfonaphthalen-2-yl)ureido)naphthalene-2-sulfonic acid | CHEMBL1092740 | NSC-47762 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C35H27N7O10S2 |
|---|
| Mol. Mass. | 769.76 |
|---|
| SMILES | CC(=O)Nc1ccc(cc1)N=Nc1c(O)c2ccc(NC(=O)Nc3ccc4c(O)c(N=Nc5ccccc5)c(cc4c3)S(O)(=O)=O)cc2cc1S(O)(=O)=O |w:31.32,11.12| |
|---|
| Structure | 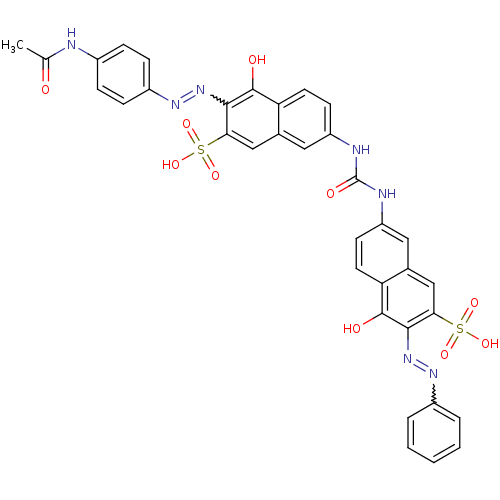
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Feng, Y; Li, M; Wang, B; Zheng, YG Discovery and mechanistic study of a class of protein arginine methylation inhibitors. J Med Chem53:6028-39 (2010) [PubMed] Article
Feng, Y; Li, M; Wang, B; Zheng, YG Discovery and mechanistic study of a class of protein arginine methylation inhibitors. J Med Chem53:6028-39 (2010) [PubMed] Article