| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glycogen synthase kinase-3 beta |
|---|
| Ligand | BDBM50332532 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_695103 (CHEMBL1640851) |
|---|
| IC50 | 214±n/a nM |
|---|
| Citation |  Khanfar, MA; Hill, RA; Kaddoumi, A; El Sayed, KA Discovery of novel GSK-3ß inhibitors with potent in vitro and in vivo activities and excellent brain permeability using combined ligand- and structure-based virtual screening. J Med Chem53:8534-45 (2010) [PubMed] Article Khanfar, MA; Hill, RA; Kaddoumi, A; El Sayed, KA Discovery of novel GSK-3ß inhibitors with potent in vitro and in vivo activities and excellent brain permeability using combined ligand- and structure-based virtual screening. J Med Chem53:8534-45 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glycogen synthase kinase-3 beta |
|---|
| Name: | Glycogen synthase kinase-3 beta |
|---|
| Synonyms: | GSK-3 beta | GSK-3, beta | GSK3B | GSK3B_HUMAN | Glycogen synthase kinase 3 beta (GSK3B) | Glycogen synthase kinase 3-beta (GSK3B) | Glycogen synthase kinase-3 beta (GSK-3B) | Glycogen synthase kinase-3 beta (GSK3 Beta) | Glycogen synthase kinase-3 beta (GSK3B) | Glycogen synthase kinase-3B (GSK-3B) | Glycogen synthase kinase-3beta (GSK3B) | Serine/threonine-protein kinase GSK3B |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 46756.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P49841 |
|---|
| Residue: | 420 |
|---|
| Sequence: | MSGRPRTTSFAESCKPVQQPSAFGSMKVSRDKDGSKVTTVVATPGQGPDRPQEVSYTDTK
VIGNGSFGVVYQAKLCDSGELVAIKKVLQDKRFKNRELQIMRKLDHCNIVRLRYFFYSSG
EKKDEVYLNLVLDYVPETVYRVARHYSRAKQTLPVIYVKLYMYQLFRSLAYIHSFGICHR
DIKPQNLLLDPDTAVLKLCDFGSAKQLVRGEPNVSYICSRYYRAPELIFGATDYTSSIDV
WSAGCVLAELLLGQPIFPGDSGVDQLVEIIKVLGTPTREQIREMNPNYTEFKFPQIKAHP
WTKVFRPRTPPEAIALCSRLLEYTPTARLTPLEACAHSFFDELRDPNVKLPNGRDTPALF
NFTTQELSSNPPLATILIPPHARIQAAASTPTNATAASDANTGDRGQTNNAASASASNST
|
|
|
|---|
| BDBM50332532 |
|---|
| n/a |
|---|
| Name | BDBM50332532 |
|---|
| Synonyms: | (E)-5-(3,4,5-trimethoxybenzylidene)imidazolidine-2,4-dione | CHEMBL1630442 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C13H14N2O5 |
|---|
| Mol. Mass. | 278.2607 |
|---|
| SMILES | COc1cc(\C=C2\NC(=O)NC2=O)cc(OC)c1OC |
|---|
| Structure | 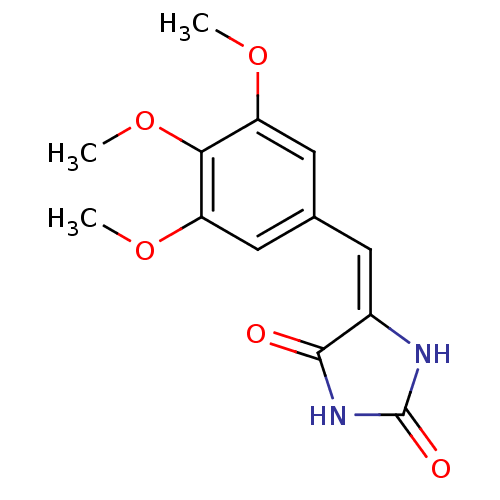
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Khanfar, MA; Hill, RA; Kaddoumi, A; El Sayed, KA Discovery of novel GSK-3ß inhibitors with potent in vitro and in vivo activities and excellent brain permeability using combined ligand- and structure-based virtual screening. J Med Chem53:8534-45 (2010) [PubMed] Article
Khanfar, MA; Hill, RA; Kaddoumi, A; El Sayed, KA Discovery of novel GSK-3ß inhibitors with potent in vitro and in vivo activities and excellent brain permeability using combined ligand- and structure-based virtual screening. J Med Chem53:8534-45 (2010) [PubMed] Article