| Reaction Details |
|---|
 | Report a problem with these data |
| Target | cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A |
|---|
| Ligand | BDBM50334653 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_699038 (CHEMBL1646319) |
|---|
| IC50 | 10000±n/a nM |
|---|
| Citation |  Asproni, B; Murineddu, G; Pau, A; Pinna, GA; Langgård, M; Christoffersen, CT; Nielsen, J; Kehler, J Synthesis and SAR study of new phenylimidazole-pyrazolo[1,5-c]quinazolines as potent phosphodiesterase 10A inhibitors. Bioorg Med Chem19:642-9 (2011) [PubMed] Article Asproni, B; Murineddu, G; Pau, A; Pinna, GA; Langgård, M; Christoffersen, CT; Nielsen, J; Kehler, J Synthesis and SAR study of new phenylimidazole-pyrazolo[1,5-c]quinazolines as potent phosphodiesterase 10A inhibitors. Bioorg Med Chem19:642-9 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A |
|---|
| Name: | cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A |
|---|
| Synonyms: | 3',5'-cyclic phosphodiesterase | CGI-PDE A | Cyclic GMP-inhibited phosphodiesterase A | PDE3A | PDE3A_HUMAN | Phosphodiesterase 3 | Phosphodiesterase 3 (PDE3) | Phosphodiesterase 3 and 5 (PDE3 and PDE5) | Phosphodiesterase 3A | Phosphodiesterase 3A (PDE3A) | Phosphodiesterase 3A (PDE3A1) | Phosphodiesterase Type 3 (PDE3A) | cGMP-inhibited 3',5'-cyclic phosphodiesterase A | cGMP-inhibited 3,5-cyclic phosphodiesterase A |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 124966.46 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q14432 |
|---|
| Residue: | 1141 |
|---|
| Sequence: | MAVPGDAARVRDKPVHSGVSQAPTAGRDCHHRADPASPRDSGCRGCWGDLVLQPLRSSRK
LSSALCAGSLSFLLALLVRLVRGEVGCDLEQCKEAAAAEEEEAAPGAEGGVFPGPRGGAP
GGGARLSPWLQPSALLFSLLCAFFWMGLYLLRAGVRLPLAVALLAACCGGEALVQIGLGV
GEDHLLSLPAAGVVLSCLAAATWLVLRLRLGVLMIALTSAVRTVSLISLERFKVAWRPYL
AYLAGVLGILLARYVEQILPQSAEAAPREHLGSQLIAGTKEDIPVFKRRRRSSSVVSAEM
SGCSSKSHRRTSLPCIPREQLMGHSEWDHKRGPRGSQSSGTSITVDIAVMGEAHGLITDL
LADPSLPPNVCTSLRAVSNLLSTQLTFQAIHKPRVNPVTSLSENYTCSDSEESSEKDKLA
IPKRLRRSLPPGLLRRVSSTWTTTTSATGLPTLEPAPVRRDRSTSIKLQEAPSSSPDSWN
NPVMMTLTKSRSFTSSYAISAANHVKAKKQSRPGALAKISPLSSPCSSPLQGTPASSLVS
KISAVQFPESADTTAKQSLGSHRALTYTQSAPDLSPQILTPPVICSSCGRPYSQGNPADE
PLERSGVATRTPSRTDDTAQVTSDYETNNNSDSSDIVQNEDETECLREPLRKASACSTYA
PETMMFLDKPILAPEPLVMDNLDSIMEQLNTWNFPIFDLVENIGRKCGRILSQVSYRLFE
DMGLFEAFKIPIREFMNYFHALEIGYRDIPYHNRIHATDVLHAVWYLTTQPIPGLSTVIN
DHGSTSDSDSDSGFTHGHMGYVFSKTYNVTDDKYGCLSGNIPALELMALYVAAAMHDYDH
PGRTNAFLVATSAPQAVLYNDRSVLENHHAAAAWNLFMSRPEYNFLINLDHVEFKHFRFL
VIEAILATDLKKHFDFVAKFNGKVNDDVGIDWTNENDRLLVCQMCIKLADINGPAKCKEL
HLQWTDGIVNEFYEQGDEEASLGLPISPFMDRSAPQLANLQESFISHIVGPLCNSYDSAG
LMPGKWVEDSDESGDTDDPEEEEEEAPAPNEEETCENNESPKKKTFKRRKIYCQITQHLL
QNHKMWKKVIEEEQRLAGIENQSLDQTPQSHSSEQIQAIKEEEEEKGKPRGEEIPTQKPD
Q
|
|
|
|---|
| BDBM50334653 |
|---|
| n/a |
|---|
| Name | BDBM50334653 |
|---|
| Synonyms: | 5-((1H-benzo[d]imidazol-2-yl)methylthio)-2-methyl-[1,2,4]triazolo[1,5-c]quinazoline | 5-(1H-BENZIMIDAZOL-2-YLMETHYLSULFANYL)-2-METHYL-[1,2,4]TRIAZOLO[1,5-C]QUINAZOLINE | CHEMBL1641615 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H14N6S |
|---|
| Mol. Mass. | 346.409 |
|---|
| SMILES | Cc1nc2c3ccccc3nc(SCc3nc4ccccc4[nH]3)n2n1 |
|---|
| Structure | 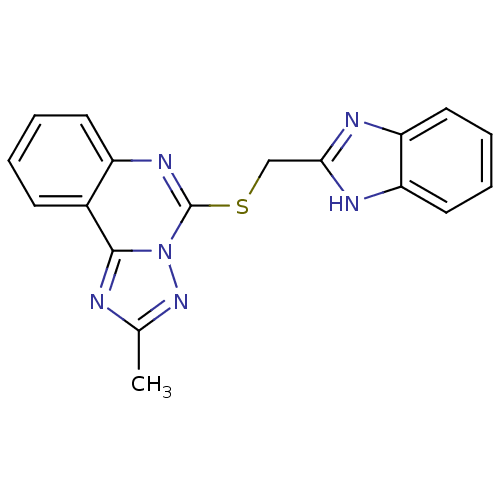
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Asproni, B; Murineddu, G; Pau, A; Pinna, GA; Langgård, M; Christoffersen, CT; Nielsen, J; Kehler, J Synthesis and SAR study of new phenylimidazole-pyrazolo[1,5-c]quinazolines as potent phosphodiesterase 10A inhibitors. Bioorg Med Chem19:642-9 (2011) [PubMed] Article
Asproni, B; Murineddu, G; Pau, A; Pinna, GA; Langgård, M; Christoffersen, CT; Nielsen, J; Kehler, J Synthesis and SAR study of new phenylimidazole-pyrazolo[1,5-c]quinazolines as potent phosphodiesterase 10A inhibitors. Bioorg Med Chem19:642-9 (2011) [PubMed] Article