| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Neuromedin-K receptor |
|---|
| Ligand | BDBM50338375 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_728272 (CHEMBL1687429) |
|---|
| EC50 | 3.3±n/a nM |
|---|
| Citation |  Xiong, H; Kang, J; Woods, JM; McCauley, JP; Koether, GM; Albert, JS; Hinkley, L; Li, Y; Gadient, RA; Simpson, TR Synthesis and SAR of sulfoxide substituted carboxyquinolines as NK3 receptor antagonists. Bioorg Med Chem Lett21:1896-9 (2011) [PubMed] Article Xiong, H; Kang, J; Woods, JM; McCauley, JP; Koether, GM; Albert, JS; Hinkley, L; Li, Y; Gadient, RA; Simpson, TR Synthesis and SAR of sulfoxide substituted carboxyquinolines as NK3 receptor antagonists. Bioorg Med Chem Lett21:1896-9 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Neuromedin-K receptor |
|---|
| Name: | Neuromedin-K receptor |
|---|
| Synonyms: | NK-3 receptor | NK-3R | NK3R | NK3R_HUMAN | NKR | Neurokinin 3 receptor | Neurokinin B receptor | Neurokinin-3 (NK-3) | Neuromedin-3 receptor (NK-3R) | Neuromedin-3 receptor (NK3) | Neuromedin-K receptor | Neuromedin-K receptor (NK-3 receptor) | Neuromedin-K receptor (NK3) | Neuromedin-K receptor(NK3R) | TAC3R | TACR3 | Tachykinin receptor 3 | Tachykinin receptor 3 (NK3) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 52221.96 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P29371 |
|---|
| Residue: | 465 |
|---|
| Sequence: | MATLPAAETWIDGGGGVGADAVNLTASLAAGAATGAVETGWLQLLDQAGNLSSSPSALGL
PVASPAPSQPWANLTNQFVQPSWRIALWSLAYGVVVAVAVLGNLIVIWIILAHKRMRTVT
NYFLVNLAFSDASMAAFNTLVNFIYALHSEWYFGANYCRFQNFFPITAVFASIYSMTAIA
VDRYMAIIDPLKPRLSATATKIVIGSIWILAFLLAFPQCLYSKTKVMPGRTLCFVQWPEG
PKQHFTYHIIVIILVYCFPLLIMGITYTIVGITLWGGEIPGDTCDKYHEQLKAKRKVVKM
MIIVVMTFAICWLPYHIYFILTAIYQQLNRWKYIQQVYLASFWLAMSSTMYNPIIYCCLN
KRFRAGFKRAFRWCPFIKVSSYDELELKTTRFHPNRQSSMYTVTRMESMTVVFDPNDADT
TRSSRKKRATPRDPSFNGCSRRNSKSASATSSFISSPYTSVDEYS
|
|
|
|---|
| BDBM50338375 |
|---|
| n/a |
|---|
| Name | BDBM50338375 |
|---|
| Synonyms: | 3-(2-(methylsulfinyl)ethyl)-2-phenyl-N-((S)-1-phenylpropyl)quinoline-4-carboxamide | CHEMBL1682945 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H28N2O2S |
|---|
| Mol. Mass. | 456.599 |
|---|
| SMILES | CC[C@H](NC(=O)c1c(CCS(C)=O)c(nc2ccccc12)-c1ccccc1)c1ccccc1 |r| |
|---|
| Structure | 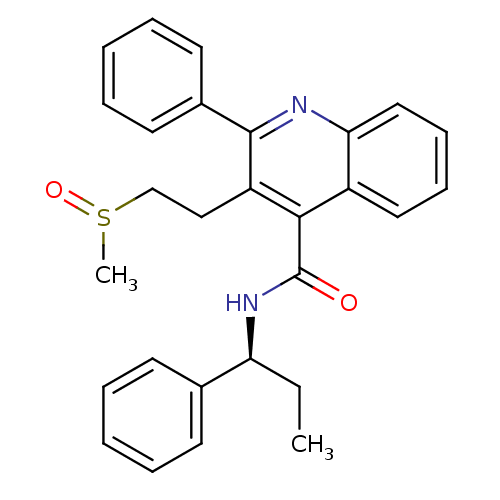
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Xiong, H; Kang, J; Woods, JM; McCauley, JP; Koether, GM; Albert, JS; Hinkley, L; Li, Y; Gadient, RA; Simpson, TR Synthesis and SAR of sulfoxide substituted carboxyquinolines as NK3 receptor antagonists. Bioorg Med Chem Lett21:1896-9 (2011) [PubMed] Article
Xiong, H; Kang, J; Woods, JM; McCauley, JP; Koether, GM; Albert, JS; Hinkley, L; Li, Y; Gadient, RA; Simpson, TR Synthesis and SAR of sulfoxide substituted carboxyquinolines as NK3 receptor antagonists. Bioorg Med Chem Lett21:1896-9 (2011) [PubMed] Article