| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Seed linoleate 13S-lipoxygenase-1 |
|---|
| Ligand | BDBM24521 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_791246 (CHEMBL1929733) |
|---|
| IC50 | 114600±n/a nM |
|---|
| Citation |  Eleftheriou, P; Geronikaki, A; Hadjipavlou-Litina, D; Vicini, P; Filz, O; Filimonov, D; Poroikov, V; Chaudhaery, SS; Roy, KK; Saxena, AK Fragment-based design, docking, synthesis, biological evaluation and structure-activity relationships of 2-benzo/benzisothiazolimino-5-aryliden-4-thiazolidinones as cycloxygenase/lipoxygenase inhibitors. Eur J Med Chem47:111-24 (2012) [PubMed] Article Eleftheriou, P; Geronikaki, A; Hadjipavlou-Litina, D; Vicini, P; Filz, O; Filimonov, D; Poroikov, V; Chaudhaery, SS; Roy, KK; Saxena, AK Fragment-based design, docking, synthesis, biological evaluation and structure-activity relationships of 2-benzo/benzisothiazolimino-5-aryliden-4-thiazolidinones as cycloxygenase/lipoxygenase inhibitors. Eur J Med Chem47:111-24 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Seed linoleate 13S-lipoxygenase-1 |
|---|
| Name: | Seed linoleate 13S-lipoxygenase-1 |
|---|
| Synonyms: | 15-LOX | 15-Lipo-oxygenase (15-LO) | 15-lipo-oxygenase (SLO) | Arachidonic Acid 15- Lipoxygenase | L-1 | LOX1 | LOX1.1 | LOX1_SOYBN | Lipoxygenase (LOX) | Lipoxygenase (SLO) | Lipoxygenase-1 | Seed lipoxygenase-1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 94365.66 |
|---|
| Organism: | Glycine max (soybean) |
|---|
| Description: | n/a |
|---|
| Residue: | 839 |
|---|
| Sequence: | MFSAGHKIKGTVVLMPKNELEVNPDGSAVDNLNAFLGRSVSLQLISATKADAHGKGKVGK
DTFLEGINTSLPTLGAGESAFNIHFEWDGSMGIPGAFYIKNYMQVEFFLKSLTLEAISNQ
GTIRFVCNSWVYNTKLYKSVRIFFANHTYVPSETPAPLVSYREEELKSLRGNGTGERKEY
DRIYDYDVYNDLGNPDKSEKLARPVLGGSSTFPYPRRGRTGRGPTVTDPNTEKQGEVFYV
PRDENLGHLKSKDALEIGTKSLSQIVQPAFESAFDLKSTPIEFHSFQDVHDLYEGGIKLP
RDVISTIIPLPVIKELYRTDGQHILKFPQPHVVQVSQSAWMTDEEFAREMIAGVNPCVIR
GLEEFPPKSNLDPAIYGDQSSKITADSLDLDGYTMDEALGSRRLFMLDYHDIFMPYVRQI
NQLNSAKTYATRTILFLREDGTLKPVAIELSLPHSAGDLSAAVSQVVLPAKEGVESTIWL
LAKAYVIVNDSCYHQLMSHWLNTHAAMEPFVIATHRHLSVLHPIYKLLTPHYRNNMNINA
LARQSLINANGIIETTFLPSKYSVEMSSAVYKNWVFTDQALPADLIKRGVAIKDPSTPHG
VRLLIEDYPYAADGLEIWAAIKTWVQEYVPLYYARDDDVKNDSELQHWWKEAVEKGHGDL
KDKPWWPKLQTLEDLVEVCLIIIWIASALHAAVNFGQYPYGGLIMNRPTASRRLLPEKGT
PEYEEMINNHEKAYLRTITSKLPTLISLSVIEILSTHASDEVYLGQRDNPHWTSDSKALQ
AFQKFGNKLKEIEEKLVRRNNDPSLQGNRLGPVQLPYTLLYPSSEEGLTFRGIPNSISI
|
|
|
|---|
| BDBM24521 |
|---|
| n/a |
|---|
| Name | BDBM24521 |
|---|
| Synonyms: | (2E,5E)-5-[(2-chlorophenyl)methylidene]-2-(1,3-thiazol-2-ylimino)-1,3-thiazolidin-4-one | Thiazole analogue, 8 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C13H8ClN3OS2 |
|---|
| Mol. Mass. | 321.805 |
|---|
| SMILES | Clc1ccccc1\C=C1\S\C(NC1=O)=N\c1nccs1 |
|---|
| Structure | 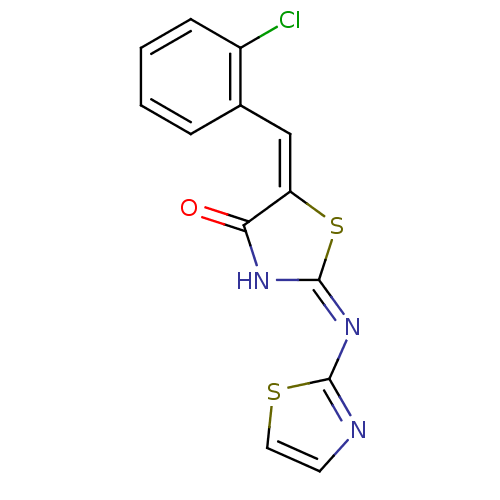
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Eleftheriou, P; Geronikaki, A; Hadjipavlou-Litina, D; Vicini, P; Filz, O; Filimonov, D; Poroikov, V; Chaudhaery, SS; Roy, KK; Saxena, AK Fragment-based design, docking, synthesis, biological evaluation and structure-activity relationships of 2-benzo/benzisothiazolimino-5-aryliden-4-thiazolidinones as cycloxygenase/lipoxygenase inhibitors. Eur J Med Chem47:111-24 (2012) [PubMed] Article
Eleftheriou, P; Geronikaki, A; Hadjipavlou-Litina, D; Vicini, P; Filz, O; Filimonov, D; Poroikov, V; Chaudhaery, SS; Roy, KK; Saxena, AK Fragment-based design, docking, synthesis, biological evaluation and structure-activity relationships of 2-benzo/benzisothiazolimino-5-aryliden-4-thiazolidinones as cycloxygenase/lipoxygenase inhibitors. Eur J Med Chem47:111-24 (2012) [PubMed] Article