

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Nitric oxide synthase, endothelial | ||
| Ligand | BDBM50365340 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_806490 (CHEMBL1959623) | ||
| IC50 | 220000±n/a nM | ||
| Citation |  Annedi, SC; Ramnauth, J; Maddaford, SP; Renton, P; Rakhit, S; Mladenova, G; Dove, P; Silverman, S; Andrews, JS; Felice, MD; Porreca, F Discovery of cis-N-(1-(4-(methylamino)cyclohexyl)indolin-6-yl)thiophene-2-carboximidamide: a 1,6-disubstituted indoline derivative as a highly selective inhibitor of human neuronal nitric oxide synthase (nNOS) without any cardiovascular liabilities. J Med Chem55:943-55 (2012) [PubMed] Article Annedi, SC; Ramnauth, J; Maddaford, SP; Renton, P; Rakhit, S; Mladenova, G; Dove, P; Silverman, S; Andrews, JS; Felice, MD; Porreca, F Discovery of cis-N-(1-(4-(methylamino)cyclohexyl)indolin-6-yl)thiophene-2-carboximidamide: a 1,6-disubstituted indoline derivative as a highly selective inhibitor of human neuronal nitric oxide synthase (nNOS) without any cardiovascular liabilities. J Med Chem55:943-55 (2012) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Nitric oxide synthase, endothelial | |||
| Name: | Nitric oxide synthase, endothelial | ||
| Synonyms: | Constitutive NOS | EC-NOS | Endothelial NOS | Endothelial nitric oxide synthase | NOS type III | NOS3 | NOS3_HUMAN | NOSIII | Nitric oxide synthase (inducible and endothelial) | Nitric oxide synthase, endothelial (eNOS) | Nitric-oxide synthase (endothelial and brain) | cNOS | eNOS | ||
| Type: | Enzyme Catalytic Domain | ||
| Mol. Mass.: | 133297.84 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P29474 | ||
| Residue: | 1203 | ||
| Sequence: |
| ||
| BDBM50365340 | |||
| n/a | |||
| Name | BDBM50365340 | ||
| Synonyms: | CHEMBL1956109 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C20H25FN4S | ||
| Mol. Mass. | 372.503 | ||
| SMILES | CN[C@H]1CC[C@H](CC1)N1CCc2cc(F)c(cc12)N=C(N)c1cccs1 |r,w:18.20,wD:5.8,2.1,(7.37,-3.67,;8.7,-2.9,;8.7,-1.36,;10.04,-.59,;10.04,.95,;8.7,1.71,;7.37,.94,;7.37,-.59,;8.71,3.26,;9.62,4.51,;8.71,5.77,;7.23,5.29,;5.9,6.05,;4.57,5.28,;3.23,6.05,;4.57,3.74,;5.9,2.97,;7.23,3.74,;3.23,2.97,;1.9,3.74,;1.9,5.28,;.57,2.97,;-.84,3.6,;-1.87,2.46,;-1.1,1.13,;.41,1.45,)| | ||
| Structure | 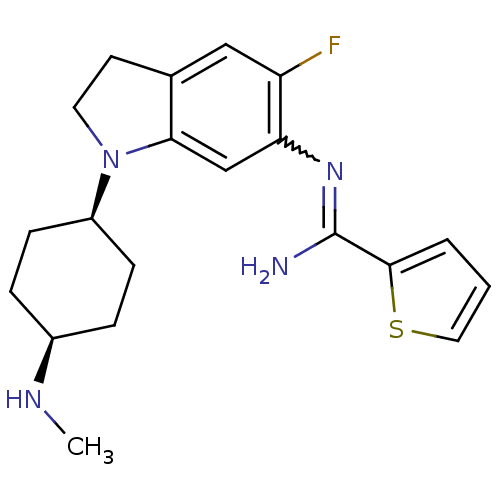
| ||