| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM50091969 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_195054 (CHEMBL798358) |
|---|
| IC50 | 1600±n/a nM |
|---|
| Citation |  Venkatachalam, TK; Sudbeck, EA; Mao, C; Uckun, FM Stereochemistry of halopyridyl and thiazolyl thiourea compounds is a major determinant of their potency as nonnucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg Med Chem Lett10:2071-4 (2000) [PubMed] Venkatachalam, TK; Sudbeck, EA; Mao, C; Uckun, FM Stereochemistry of halopyridyl and thiazolyl thiourea compounds is a major determinant of their potency as nonnucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg Med Chem Lett10:2071-4 (2000) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM50091969 |
|---|
| n/a |
|---|
| Name | BDBM50091969 |
|---|
| Synonyms: | 1-(5-Bromo-pyridin-2-yl)-3-((R)-1-phenyl-ethyl)-thiourea | CHEMBL432526 | HI-511 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H14BrN3S |
|---|
| Mol. Mass. | 336.25 |
|---|
| SMILES | C[C@@H](NC(=S)Nc1ccc(Br)cn1)c1ccccc1 |
|---|
| Structure | 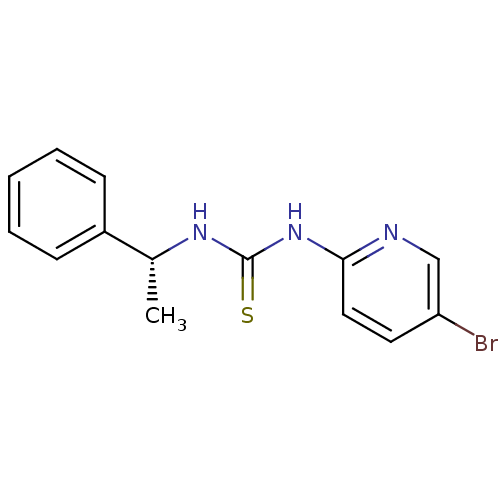
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Venkatachalam, TK; Sudbeck, EA; Mao, C; Uckun, FM Stereochemistry of halopyridyl and thiazolyl thiourea compounds is a major determinant of their potency as nonnucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg Med Chem Lett10:2071-4 (2000) [PubMed]
Venkatachalam, TK; Sudbeck, EA; Mao, C; Uckun, FM Stereochemistry of halopyridyl and thiazolyl thiourea compounds is a major determinant of their potency as nonnucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg Med Chem Lett10:2071-4 (2000) [PubMed]