| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Nitric oxide synthase, inducible |
|---|
| Ligand | BDBM50122307 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_89197 |
|---|
| IC50 | 5000±n/a nM |
|---|
| Citation |  Jaroch, S; Hölscher, P; Rehwinkel, H; Sülzle, D; Burton, G; Hillmann, M; McDonald, FM Dihydroquinolines with amine-containing side chains as potent n-NOS inhibitors. Bioorg Med Chem Lett13:1981-4 (2003) [PubMed] Jaroch, S; Hölscher, P; Rehwinkel, H; Sülzle, D; Burton, G; Hillmann, M; McDonald, FM Dihydroquinolines with amine-containing side chains as potent n-NOS inhibitors. Bioorg Med Chem Lett13:1981-4 (2003) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Nitric oxide synthase, inducible |
|---|
| Name: | Nitric oxide synthase, inducible |
|---|
| Synonyms: | HEP-NOS | Hepatocyte NOS | Inducible NO synthase | Inducible NOS | NOS type II | NOS2 | NOS2A | NOS2_HUMAN | Nitric oxide synthase, inducible (iNOS) | iNOS |
|---|
| Type: | Homodimer |
|---|
| Mol. Mass.: | 131141.95 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P35228 |
|---|
| Residue: | 1153 |
|---|
| Sequence: | MACPWKFLFKTKFHQYAMNGEKDINNNVEKAPCATSSPVTQDDLQYHNLSKQQNESPQPL
VETGKKSPESLVKLDATPLSSPRHVRIKNWGSGMTFQDTLHHKAKGILTCRSKSCLGSIM
TPKSLTRGPRDKPTPPDELLPQAIEFVNQYYGSFKEAKIEEHLARVEAVTKEIETTGTYQ
LTGDELIFATKQAWRNAPRCIGRIQWSNLQVFDARSCSTAREMFEHICRHVRYSTNNGNI
RSAITVFPQRSDGKHDFRVWNAQLIRYAGYQMPDGSIRGDPANVEFTQLCIDLGWKPKYG
RFDVVPLVLQANGRDPELFEIPPDLVLEVAMEHPKYEWFRELELKWYALPAVANMLLEVG
GLEFPGCPFNGWYMGTEIGVRDFCDVQRYNILEEVGRRMGLETHKLASLWKDQAVVEINI
AVLHSFQKQNVTIMDHHSAAESFMKYMQNEYRSRGGCPADWIWLVPPMSGSITPVFHQEM
LNYVLSPFYYYQVEAWKTHVWQDEKRRPKRREIPLKVLVKAVLFACMLMRKTMASRVRVT
ILFATETGKSEALAWDLGALFSCAFNPKVVCMDKYRLSCLEEERLLLVVTSTFGNGDCPG
NGEKLKKSLFMLKELNNKFRYAVFGLGSSMYPRFCAFAHDIDQKLSHLGASQLTPMGEGD
ELSGQEDAFRSWAVQTFKAACETFDVRGKQHIQIPKLYTSNVTWDPHHYRLVQDSQPLDL
SKALSSMHAKNVFTMRLKSRQNLQSPTSSRATILVELSCEDGQGLNYLPGEHLGVCPGNQ
PALVQGILERVVDGPTPHQTVRLEALDESGSYWVSDKRLPPCSLSQALTYFLDITTPPTQ
LLLQKLAQVATEEPERQRLEALCQPSEYSKWKFTNSPTFLEVLEEFPSLRVSAGFLLSQL
PILKPRFYSISSSRDHTPTEIHLTVAVVTYHTRDGQGPLHHGVCSTWLNSLKPQDPVPCF
VRNASGFHLPEDPSHPCILIGPGTGIAPFRSFWQQRLHDSQHKGVRGGRMTLVFGCRRPD
EDHIYQEEMLEMAQKGVLHAVHTAYSRLPGKPKVYVQDILRQQLASEVLRVLHKEPGHLY
VCGDVRMARDVAHTLKQLVAAKLKLNEEQVEDYFFQLKSQKRYHEDIFGAVFPYEAKKDR
VAVQPSSLEMSAL
|
|
|
|---|
| BDBM50122307 |
|---|
| n/a |
|---|
| Name | BDBM50122307 |
|---|
| Synonyms: | CHEMBL293212 | N-{4-[2-(3-Chloro-benzylamino)-ethyl]-phenyl}-thiophene-2-carboxamidine; hydrochloride |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H20ClN3S |
|---|
| Mol. Mass. | 369.911 |
|---|
| SMILES | NC(=Nc1ccc(CCNCc2cccc(Cl)c2)cc1)c1cccs1 |w:2.2| |
|---|
| Structure | 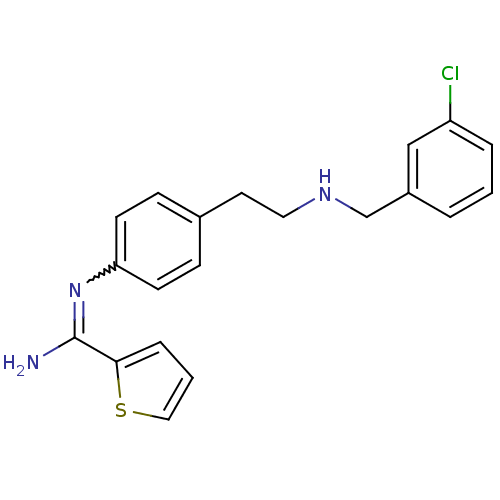
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Jaroch, S; Hölscher, P; Rehwinkel, H; Sülzle, D; Burton, G; Hillmann, M; McDonald, FM Dihydroquinolines with amine-containing side chains as potent n-NOS inhibitors. Bioorg Med Chem Lett13:1981-4 (2003) [PubMed]
Jaroch, S; Hölscher, P; Rehwinkel, H; Sülzle, D; Burton, G; Hillmann, M; McDonald, FM Dihydroquinolines with amine-containing side chains as potent n-NOS inhibitors. Bioorg Med Chem Lett13:1981-4 (2003) [PubMed]