| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Endothelin receptor type B |
|---|
| Ligand | BDBM50368606 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_64189 (CHEMBL676550) |
|---|
| Ki | 0.012000±n/a nM |
|---|
| Citation |  Doherty, AM Endothelin: a new challenge. J Med Chem35:1493-508 (1992) [PubMed] Doherty, AM Endothelin: a new challenge. J Med Chem35:1493-508 (1992) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Endothelin receptor type B |
|---|
| Name: | Endothelin receptor type B |
|---|
| Synonyms: | EDNRB_RAT | ENDOTHELIN B | ET-B | Ednrb | Endothelin receptor | Endothelin receptor non-selective type |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 49483.43 |
|---|
| Organism: | RAT |
|---|
| Description: | ENDOTHELIN B EDNRB RAT::P21451 |
|---|
| Residue: | 442 |
|---|
| Sequence: | MQSSASRCGRALVALLLACGLLGVWGEKRGFPPAQATPSLLGTKEVMTPPTKTSWTRGSN
SSLMRSSAPAEVTKGGRVAGVPPRSFPPPCQRKIEINKTFKYINTIVSCLVFVLGIIGNS
TLLRIIYKNKCMRNGPNILIASLALGDLLHIIIDIPINAYKLLAGDWPFGAEMCKLVPFI
QKASVGITVLSLCALSIDRYRAVASWSRIKGIGVPKWTAVEIVLIWVVSVVLAVPEAIGF
DVITSDYKGKPLRVCMLNPFQKTAFMQFYKTAKDWWLFSFYFCLPLAITAIFYTLMTCEM
LRKKSGMQIALNDHLKQRREVAKTVFCLVLVFALCWLPLHLSRILKLTLYDQSNPQRCEL
LSFLLVLDYIGINMASLNSCINPIALYLVSKRFKNCFKSCLCCWCQTFEEKQSLEEKQSC
LKFKANDHGYDNFRSSNKYSSS
|
|
|
|---|
| BDBM50368606 |
|---|
| n/a |
|---|
| Name | BDBM50368606 |
|---|
| Synonyms: | Sarafotoxin S6B |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C110H159N27O34S5 |
|---|
| Mol. Mass. | 2563.925 |
|---|
| SMILES | [H][C@]12CSSC[C@]([H])(NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@]([H])(NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC1=O)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N2)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| |
|---|
| Structure | 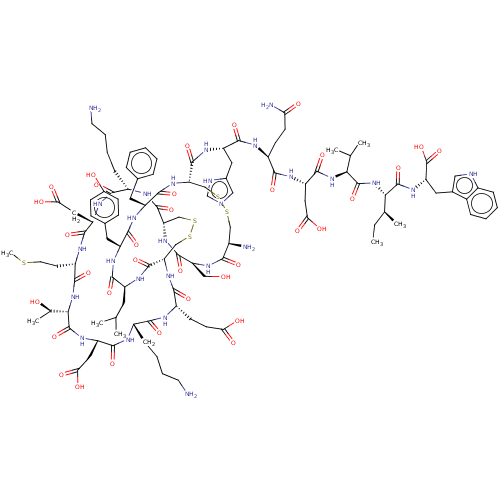
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Doherty, AM Endothelin: a new challenge. J Med Chem35:1493-508 (1992) [PubMed]
Doherty, AM Endothelin: a new challenge. J Med Chem35:1493-508 (1992) [PubMed]