| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 3-oxo-5-alpha-steroid 4-dehydrogenase 2 |
|---|
| Ligand | BDBM50368782 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_204740 (CHEMBL805433) |
|---|
| IC50 | 1750±n/a nM |
|---|
| Citation |  Bakshi, RK; Rasmusson, GH; Patel, GF; Mosley, RT; Chang, B; Ellsworth, K; Harris, GS; Tolman, RL 4-Aza-3-oxo-5 alpha-androst-1-ene-17 beta-N-aryl-carboxamides as dual inhibitors of human type 1 and type 2 steroid 5 alpha-reductases. Dramatic effect of N-aryl substituents on type 1 and type 2 5 alpha-reductase inhibitory potency. J Med Chem38:3189-92 (1995) [PubMed] Bakshi, RK; Rasmusson, GH; Patel, GF; Mosley, RT; Chang, B; Ellsworth, K; Harris, GS; Tolman, RL 4-Aza-3-oxo-5 alpha-androst-1-ene-17 beta-N-aryl-carboxamides as dual inhibitors of human type 1 and type 2 steroid 5 alpha-reductases. Dramatic effect of N-aryl substituents on type 1 and type 2 5 alpha-reductase inhibitory potency. J Med Chem38:3189-92 (1995) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 |
|---|
| Name: | 3-oxo-5-alpha-steroid 4-dehydrogenase 2 |
|---|
| Synonyms: | 3-oxo-5-alpha-steroid 4-dehydrogenase 2 | 5 alpha-SR2 | 5α-Reductase 2 (5α-R2) | S5A2_HUMAN | SR type 2 | SRD5A2 | Steroid 5-alpha-reductase | Steroid 5-alpha-reductase 2 | Type II 5-alpha reductase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 28406.59 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P31213 |
|---|
| Residue: | 254 |
|---|
| Sequence: | MQVQCQQSPVLAGSATLVALGALALYVAKPSGYGKHTESLKPAATRLPARAAWFLQELPS
FAVPAGILARQPLSLFGPPGTVLLGLFCLHYFHRTFVYSLLNRGRPYPAILILRGTAFCT
GNGVLQGYYLIYCAEYPDGWYTDIRFSLGVFLFILGMGINIHSDYILRQLRKPGEISYRI
PQGGLFTYVSGANFLGEIIEWIGYALATWSLPALAFAFFSLCFLGLRAFHHHRFYLKMFE
DYPKSRKALIPFIF
|
|
|
|---|
| BDBM50368782 |
|---|
| n/a |
|---|
| Name | BDBM50368782 |
|---|
| Synonyms: | Bexlosteride | CHEMBL24955 | LY-191704 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H16ClNO |
|---|
| Mol. Mass. | 249.736 |
|---|
| SMILES | CN1[C@@H]2CCc3cc(Cl)ccc3[C@H]2CCC1=O |
|---|
| Structure | 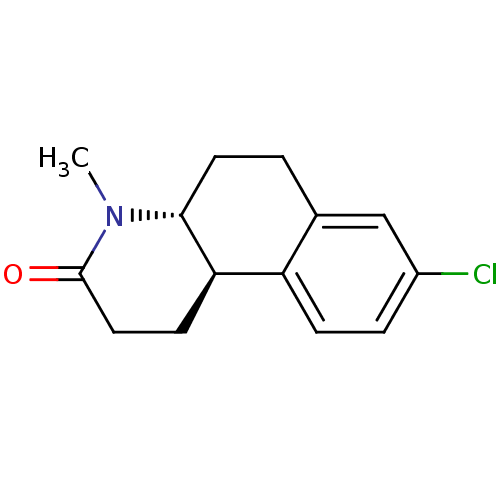
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bakshi, RK; Rasmusson, GH; Patel, GF; Mosley, RT; Chang, B; Ellsworth, K; Harris, GS; Tolman, RL 4-Aza-3-oxo-5 alpha-androst-1-ene-17 beta-N-aryl-carboxamides as dual inhibitors of human type 1 and type 2 steroid 5 alpha-reductases. Dramatic effect of N-aryl substituents on type 1 and type 2 5 alpha-reductase inhibitory potency. J Med Chem38:3189-92 (1995) [PubMed]
Bakshi, RK; Rasmusson, GH; Patel, GF; Mosley, RT; Chang, B; Ellsworth, K; Harris, GS; Tolman, RL 4-Aza-3-oxo-5 alpha-androst-1-ene-17 beta-N-aryl-carboxamides as dual inhibitors of human type 1 and type 2 steroid 5 alpha-reductases. Dramatic effect of N-aryl substituents on type 1 and type 2 5 alpha-reductase inhibitory potency. J Med Chem38:3189-92 (1995) [PubMed]