| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Phospholipase A2 |
|---|
| Ligand | BDBM50055421 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_156185 (CHEMBL760954) |
|---|
| IC50 | >100000000±n/a nM |
|---|
| Citation |  Teshirogi, I; Matsutani, S; Shirahase, K; Fujii, Y; Yoshida, T; Tanaka, K; Ohtani, M Synthesis and phospholipase A2 inhibitory activity of thielocin B3 derivatives. J Med Chem39:5183-91 (1997) [PubMed] Article Teshirogi, I; Matsutani, S; Shirahase, K; Fujii, Y; Yoshida, T; Tanaka, K; Ohtani, M Synthesis and phospholipase A2 inhibitory activity of thielocin B3 derivatives. J Med Chem39:5183-91 (1997) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Phospholipase A2 |
|---|
| Name: | Phospholipase A2 |
|---|
| Synonyms: | Group IB phospholipase A2 | PA21B_HUMAN | PLA2 | PLA2A | PLA2G1B | PPLA2 | Phosphatidylcholine 2-acylhydrolase 1B | Phospholipase A2 (PLA2) | Phospholipase A2 group 1B | phospholipase A2 precursor |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 16364.13 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P04054 |
|---|
| Residue: | 148 |
|---|
| Sequence: | MKLLVLAVLLTVAAADSGISPRAVWQFRKMIKCVIPGSDPFLEYNNYGCYCGLGGSGTPV
DELDKCCQTHDNCYDQAKKLDSCKFLLDNPYTHTYSYSCSGSAITCSSKNKECEAFICNC
DRNAAICFSKAPYNKAHKNLDTKKYCQS
|
|
|
|---|
| BDBM50055421 |
|---|
| n/a |
|---|
| Name | BDBM50055421 |
|---|
| Synonyms: | Bis[3-[[4-[[4-[[[(pivaloyloxy)methyl]carbonyl]-3-methoxy-2,5,6-trimethylphenoxy]carbonyl]-3-methoxy-2,5,6-triimethylphenoxy]carbonyl]-4-methoxyphenyl]sulfide | CHEMBL406947 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C66H72O20S |
|---|
| Mol. Mass. | 1217.331 |
|---|
| SMILES | COc1ccc(Sc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(=O)Oc3c(C)c(C)c(C(=O)OCOC(=O)C(C)(C)C)c(OC)c3C)c(OC)c2C)cc1C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C |
|---|
| Structure | 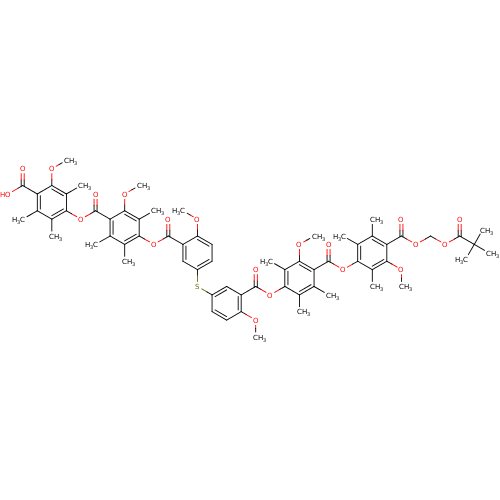
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Teshirogi, I; Matsutani, S; Shirahase, K; Fujii, Y; Yoshida, T; Tanaka, K; Ohtani, M Synthesis and phospholipase A2 inhibitory activity of thielocin B3 derivatives. J Med Chem39:5183-91 (1997) [PubMed] Article
Teshirogi, I; Matsutani, S; Shirahase, K; Fujii, Y; Yoshida, T; Tanaka, K; Ohtani, M Synthesis and phospholipase A2 inhibitory activity of thielocin B3 derivatives. J Med Chem39:5183-91 (1997) [PubMed] Article