| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Phospholipase A2, membrane associated |
|---|
| Ligand | BDBM50250399 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_156206 (CHEMBL767149) |
|---|
| IC50 | 3900±n/a nM |
|---|
| Citation |  De Rosa, M; Giordano, S; Scettri, A; Sodano, G; Soriente, A; Pastor, PG; Alcaraz, MJ; Payá, M Synthesis and comparison of the antiinflammatory activity of manoalide and cacospongionolide B analogues. J Med Chem41:3232-8 (1998) [PubMed] Article De Rosa, M; Giordano, S; Scettri, A; Sodano, G; Soriente, A; Pastor, PG; Alcaraz, MJ; Payá, M Synthesis and comparison of the antiinflammatory activity of manoalide and cacospongionolide B analogues. J Med Chem41:3232-8 (1998) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Phospholipase A2, membrane associated |
|---|
| Name: | Phospholipase A2, membrane associated |
|---|
| Synonyms: | GIIC sPLA2 | Group IIA phospholipase A2 | NPS-PLA2 | Non-Pancreatic Secretory Phospholipase A2 | Non-pancreatic secretory phospholipase A2 (hnps-PLA2) | PA2GA_HUMAN | PLA2B | PLA2G2A | PLA2L | Phosphatidylcholine 2-acylhydrolase | Phospholipase A2 group IIA | RASF-A |
|---|
| Type: | Hydrolase |
|---|
| Mol. Mass.: | 16101.20 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | The human nps PLA2 was cloned, and expressed in E. coli. There was a refolding process in the purification. |
|---|
| Residue: | 144 |
|---|
| Sequence: | MKTLLLLAVIMIFGLLQAHGNLVNFHRMIKLTTGKEAALSYGFYGCHCGVGGRGSPKDAT
DRCCVTHDCCYKRLEKRGCGTKFLSYKFSNSGSRITCAKQDSCRSQLCECDKAAATCFAR
NKTTYNKKYQYYSNKHCRGSTPRC
|
|
|
|---|
| BDBM50250399 |
|---|
| n/a |
|---|
| Name | BDBM50250399 |
|---|
| Synonyms: | 5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,6-trimethyl-cyclohex-1-enyl)-hex-3-enyl]-3,6-dihydro-2H-pyran-2-yl}-5H-furan-2-one | 5-Hydroxy-4-{6-hydroxy-5-[(E)-4-methyl-6-(2,6,6-trimethyl-cyclohex-1-enyl)-hex-3-enyl]-3,6-dihydro-2H-pyran-2-yl}-5H-furan-2-one | CHEMBL463914 | manoalide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H36O5 |
|---|
| Mol. Mass. | 416.5503 |
|---|
| SMILES | C\C(CCC1=C(C)CCCC1(C)C)=C/CCC1=CC[C@@H](O[C@H]1O)C1=CC(=O)O[C@H]1O |r,c:4,t:17,25| |
|---|
| Structure | 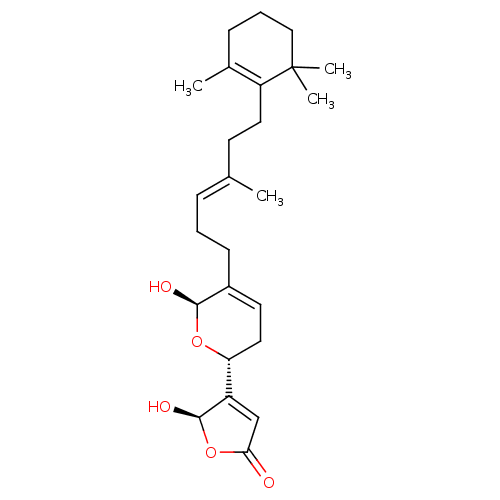
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 De Rosa, M; Giordano, S; Scettri, A; Sodano, G; Soriente, A; Pastor, PG; Alcaraz, MJ; Payá, M Synthesis and comparison of the antiinflammatory activity of manoalide and cacospongionolide B analogues. J Med Chem41:3232-8 (1998) [PubMed] Article
De Rosa, M; Giordano, S; Scettri, A; Sodano, G; Soriente, A; Pastor, PG; Alcaraz, MJ; Payá, M Synthesis and comparison of the antiinflammatory activity of manoalide and cacospongionolide B analogues. J Med Chem41:3232-8 (1998) [PubMed] Article