| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Neuraminidase |
|---|
| Ligand | BDBM5024 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_144590 (CHEMBL882330) |
|---|
| IC50 | 1.4±n/a nM |
|---|
| Citation |  Babu, YS; Chand, P; Bantia, S; Kotian, P; Dehghani, A; El-Kattan, Y; Lin, TH; Hutchison, TL; Elliott, AJ; Parker, CD; Ananth, SL; Horn, LL; Laver, GW; Montgomery, JA BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem43:3482-6 (2000) [PubMed] Babu, YS; Chand, P; Bantia, S; Kotian, P; Dehghani, A; El-Kattan, Y; Lin, TH; Hutchison, TL; Elliott, AJ; Parker, CD; Ananth, SL; Horn, LL; Laver, GW; Montgomery, JA BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem43:3482-6 (2000) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Neuraminidase |
|---|
| Name: | Neuraminidase |
|---|
| Synonyms: | Influenza A Virus Neuraminidase | NA | NRAM_I34A1 | Neuraminidase | Neuraminidase A |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 50124.14 |
|---|
| Organism: | Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(H1N1)) |
|---|
| Description: | P03468 |
|---|
| Residue: | 454 |
|---|
| Sequence: | MNPNQKIITIGSICLVVGLISLILQIGNIISIWISHSIQTGSQNHTGICNQNIITYKNST
WVKDTTSVILTGNSSLCPIRGWAIYSKDNSIRIGSKGDVFVIREPFISCSHLECRTFFLT
QGALLNDKHSNGTVKDRSPYRALMSCPVGEAPSPYNSRFESVAWSASACHDGMGWLTIGI
SGPDNGAVAVLKYNGIITETIKSWRKKILRTQESECACVNGSCFTIMTDGPSDGLASYKI
FKIEKGKVTKSIELNAPNSHYEECSCYPDTGKVMCVCRDNWHGSNRPWVSFDQNLDYQIG
YICSGVFGDNPRPEDGTGSCGPVYVDGANGVKGFSYRYGNGVWIGRTKSHSSRHGFEMIW
DPNGWTETDSKFSVRQDVVAMTDWSGYSGSFVQHPELTGLDCMRPCFWVELIRGRPKEKT
IWTSASSISFCGVNSDTVDWSWPDGAELPFSIDK
|
|
|
|---|
| BDBM5024 |
|---|
| n/a |
|---|
| Name | BDBM5024 |
|---|
| Synonyms: | (-)-(1S,2S,3R,4R)-3-[(1S)-1-(Acetylamino)-2-ethylbutyl]-4-{[amino(imino)methyl]amino}-2-hydroxycyclopentanecarboxylic Acid | (1S,2S,3R,4R)-4-carbamimidamido-3-[(1S)-1-acetamido-2-ethylbutyl]-2-hydroxycyclopentane-1-carboxylic acid | BCX-1812 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H28N4O4 |
|---|
| Mol. Mass. | 328.4072 |
|---|
| SMILES | [H][C@](NC(C)=O)(C(CC)CC)[C@@H]1[C@H](O)[C@H](C[C@H]1N=C(N)N)C(O)=O |r| |
|---|
| Structure | 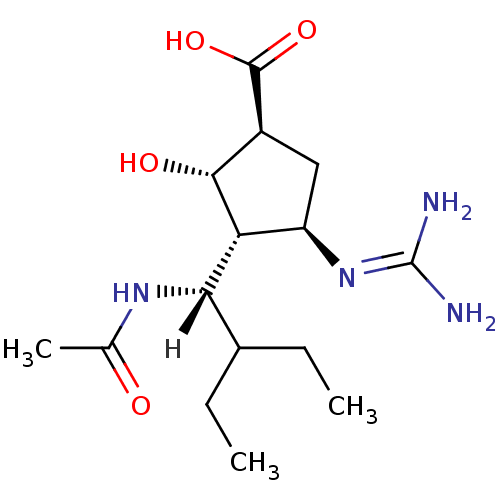
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Babu, YS; Chand, P; Bantia, S; Kotian, P; Dehghani, A; El-Kattan, Y; Lin, TH; Hutchison, TL; Elliott, AJ; Parker, CD; Ananth, SL; Horn, LL; Laver, GW; Montgomery, JA BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem43:3482-6 (2000) [PubMed]
Babu, YS; Chand, P; Bantia, S; Kotian, P; Dehghani, A; El-Kattan, Y; Lin, TH; Hutchison, TL; Elliott, AJ; Parker, CD; Ananth, SL; Horn, LL; Laver, GW; Montgomery, JA BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem43:3482-6 (2000) [PubMed]