

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Bifunctional purine biosynthesis protein ATIC | ||
| Ligand | BDBM50005518 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_353451 (CHEMBL861363) | ||
| Ki | >100000±n/a nM | ||
| Citation |  DeMartino, JK; Hwang, I; Xu, L; Wilson, IA; Boger, DL Discovery of a potent, nonpolyglutamatable inhibitor of glycinamide ribonucleotide transformylase. J Med Chem49:2998-3002 (2006) [PubMed] Article DeMartino, JK; Hwang, I; Xu, L; Wilson, IA; Boger, DL Discovery of a potent, nonpolyglutamatable inhibitor of glycinamide ribonucleotide transformylase. J Med Chem49:2998-3002 (2006) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Bifunctional purine biosynthesis protein ATIC | |||
| Name: | Bifunctional purine biosynthesis protein ATIC | ||
| Synonyms: | 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase | 5-aminoimidazole-4-carboxamide-ribonucleotide transformylase | AICAR Tfase | AICAR transformylase | ATIC | Aminoimidazole carboxamide ribonucleotide transformylase (AICAR Tfase) | Bifunctional purine biosynthesis protein PURH | IMP Cyclohydrolase (IMPCH) | IMP cyclohydrolase | IMP synthetase | Inosinicase | PUR9_HUMAN | PURH | Phosphoribosylaminoimidazolecarboxamide formyltransferase | Thymidylate synthase/GAR transformylase/AICAR transformylase | ||
| Type: | Protein | ||
| Mol. Mass.: | 64616.62 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P31939 | ||
| Residue: | 592 | ||
| Sequence: |
| ||
| BDBM50005518 | |||
| n/a | |||
| Name | BDBM50005518 | ||
| Synonyms: | (S)-2-(4-(2-((R)-2-amino-4-oxo-1,4,5,6,7,8-hexahydropyrido[2,3-d]pyrimidin-6-yl)ethyl)benzamido)pentanedioic acid | (S)-2-(4-(2-((R)-2-amino-4-oxo-3,4,5,6,7,8-hexahydropyrido[2,3-d]pyrimidin-6-yl)ethyl)benzamido)pentanedioic acid | (S)-2-{(R)-4-[2-((R)-2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[2,3-d]pyrimidin-6-yl)-ethyl]-benzoylamino}-pentanedioic acid | (S)-2-{(R)-4-[2-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[2,3-d]pyrimidin-6-yl)-ethyl]-benzoylamino}-pentanedioic acid | (S)-2-{(S)-4-[2-(2-Amino-4-hydroxy-5,6,7,8-tetrahydro-pyrido[2,3-d]pyrimidin-6-yl)-ethyl]-benzoylamino}-pentanedioic acid | (S)-2-{4-[2-((R)-2-Amino-4-oxo-1,4,5,6,7,8-hexahydro-pyrido[2,3-d]pyrimidin-6-yl)-ethyl]-benzoylamino}-pentanedioic acid | (S)-2-{4-[2-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[2,3-d]pyrimidin-6-yl)-ethyl]-benzoylamino}-pentanedioic acid | 2-{4-[2-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[2,3-d]pyrimidin-6-yl)-ethyl]-benzoylamino}-pentanedioic acid | CHEMBL142806 | LOMETREXOL | ||
| Type | Small organic molecule | ||
| Emp. Form. | C21H25N5O6 | ||
| Mol. Mass. | 443.4531 | ||
| SMILES | Nc1nc2NCC(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)Cc2c(=O)[nH]1 |r| | ||
| Structure | 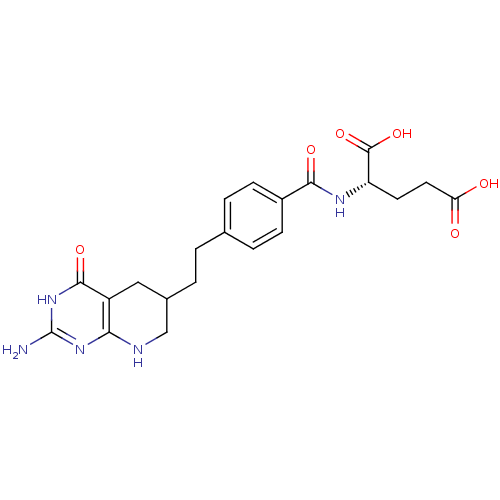
| ||