| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prostaglandin G/H synthase 2 |
|---|
| Ligand | BDBM50373577 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_468017 (CHEMBL947228) |
|---|
| IC50 | 9.2±n/a nM |
|---|
| Citation |  Orjales, A; Mosquera, R; López, B; Olivera, R; Labeaga, L; Núñez, MT Novel 2-(4-methylsulfonylphenyl)pyrimidine derivatives as highly potent and specific COX-2 inhibitors. Bioorg Med Chem16:2183-99 (2008) [PubMed] Article Orjales, A; Mosquera, R; López, B; Olivera, R; Labeaga, L; Núñez, MT Novel 2-(4-methylsulfonylphenyl)pyrimidine derivatives as highly potent and specific COX-2 inhibitors. Bioorg Med Chem16:2183-99 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prostaglandin G/H synthase 2 |
|---|
| Name: | Prostaglandin G/H synthase 2 |
|---|
| Synonyms: | COX2 | Cyclooxygenase | Cyclooxygenase 2 (COX-2) | Cyclooxygenase-2 | Cyclooxygenase-2 (COX-2 AA) | Cyclooxygenase-2 (COX-2 AEA) | Cyclooxygenase-2 (COX-2) | PGH synthase 2 | PGH2_HUMAN | PGHS-2 | PHS II | PTGS2 | Prostaglandin E synthase/G/H synthase 2 | Prostaglandin H2 synthase 2 | Prostaglandin-endoperoxide synthase 2 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 69003.89 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Recombinant Cox-2 provided by Cayman (Cayman Chemical Co.,Ann Arbor, MI). |
|---|
| Residue: | 604 |
|---|
| Sequence: | MLARALLLCAVLALSHTANPCCSHPCQNRGVCMSVGFDQYKCDCTRTGFYGENCSTPEFL
TRIKLFLKPTPNTVHYILTHFKGFWNVVNNIPFLRNAIMSYVLTSRSHLIDSPPTYNADY
GYKSWEAFSNLSYYTRALPPVPDDCPTPLGVKGKKQLPDSNEIVEKLLLRRKFIPDPQGS
NMMFAFFAQHFTHQFFKTDHKRGPAFTNGLGHGVDLNHIYGETLARQRKLRLFKDGKMKY
QIIDGEMYPPTVKDTQAEMIYPPQVPEHLRFAVGQEVFGLVPGLMMYATIWLREHNRVCD
VLKQEHPEWGDEQLFQTSRLILIGETIKIVIEDYVQHLSGYHFKLKFDPELLFNKQFQYQ
NRIAAEFNTLYHWHPLLPDTFQIHDQKYNYQQFIYNNSILLEHGITQFVESFTRQIAGRV
AGGRNVPPAVQKVSQASIDQSRQMKYQSFNEYRKRFMLKPYESFEELTGEKEMSAELEAL
YGDIDAVELYPALLVEKPRPDAIFGETMVEVGAPFSLKGLMGNVICSPAYWKPSTFGGEV
GFQIINTASIQSLICNNVKGCPFTSFSVPDPELIKTVTINASSSRSGLDDINPTVLLKER
STEL
|
|
|
|---|
| BDBM50373577 |
|---|
| n/a |
|---|
| Name | BDBM50373577 |
|---|
| Synonyms: | CHEMBL405564 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H15N3O2S2 |
|---|
| Mol. Mass. | 345.439 |
|---|
| SMILES | CS(=O)(=O)c1ccc(cc1)-c1nccc(NCc2cccs2)n1 |
|---|
| Structure | 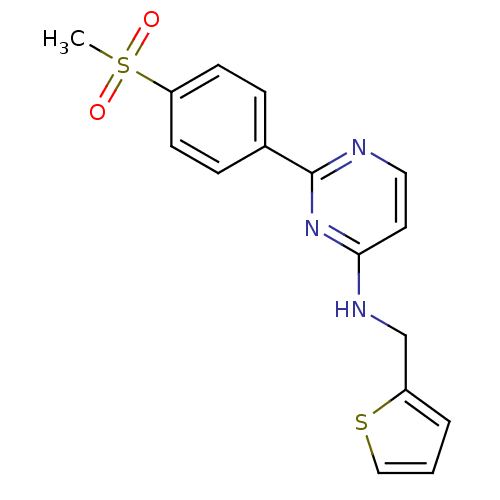
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Orjales, A; Mosquera, R; López, B; Olivera, R; Labeaga, L; Núñez, MT Novel 2-(4-methylsulfonylphenyl)pyrimidine derivatives as highly potent and specific COX-2 inhibitors. Bioorg Med Chem16:2183-99 (2008) [PubMed] Article
Orjales, A; Mosquera, R; López, B; Olivera, R; Labeaga, L; Núñez, MT Novel 2-(4-methylsulfonylphenyl)pyrimidine derivatives as highly potent and specific COX-2 inhibitors. Bioorg Med Chem16:2183-99 (2008) [PubMed] Article