| Reaction Details |
|---|
 | Report a problem with these data |
| Target | IAG-nucleoside hydrolase |
|---|
| Ligand | BDBM50260480 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_531315 (CHEMBL989727) |
|---|
| Ki | 4500000±n/a nM |
|---|
| Citation |  Goeminne, A; McNaughton, M; Bal, G; Surpateanu, G; Van Der Veken, P; De Prol, S; Versées, W; Steyaert, J; Haemers, A; Augustyns, K Synthesis and biochemical evaluation of guanidino-alkyl-ribitol derivatives as nucleoside hydrolase inhibitors. Eur J Med Chem43:315-26 (2008) [PubMed] Article Goeminne, A; McNaughton, M; Bal, G; Surpateanu, G; Van Der Veken, P; De Prol, S; Versées, W; Steyaert, J; Haemers, A; Augustyns, K Synthesis and biochemical evaluation of guanidino-alkyl-ribitol derivatives as nucleoside hydrolase inhibitors. Eur J Med Chem43:315-26 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| IAG-nucleoside hydrolase |
|---|
| Name: | IAG-nucleoside hydrolase |
|---|
| Synonyms: | n/a |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 36316.77 |
|---|
| Organism: | Trypanosoma vivax |
|---|
| Description: | ChEMBL_531315 |
|---|
| Residue: | 327 |
|---|
| Sequence: | MAKNVVLDHDGNLDDFVAMVLLASNTEKVRLIGALCTDADCFVENGFNVTGKIMCLMHNN
MNLPLFPIGKSAATAVNPFPKEWRCLAKNMDDMPILNIPENVELWDKIKAENEKYEGQQL
LADLVMNSEEKVTICVTGPLSNVAWCIDKYGEKFTSKVEECVIMGGAVDVRGNVFLPSTD
GTAEWNIYWDPASAKTVFGCPGLRRIMFSLDSTNTVPVRSPYVQRFGEQTNFLLSILVGT
MWAMCTHCELLRDGDGYYAWDALTAAYVVDQKVANVDPVPIDVVVDKQPNEGATVRTDAE
KYPLTFVARNPEAEFFLDMLLRSARAC
|
|
|
|---|
| BDBM50260480 |
|---|
| n/a |
|---|
| Name | BDBM50260480 |
|---|
| Synonyms: | (2R,3S,4R,5R)-3,4-Dihydroxy-5-(hydroxymethyl)-tetrahydro-2-furancarboxamide | CHEMBL455563 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C6H11NO5 |
|---|
| Mol. Mass. | 177.1552 |
|---|
| SMILES | NC(=O)[C@@H]1O[C@H](CO)[C@H](O)[C@@H]1O |r| |
|---|
| Structure | 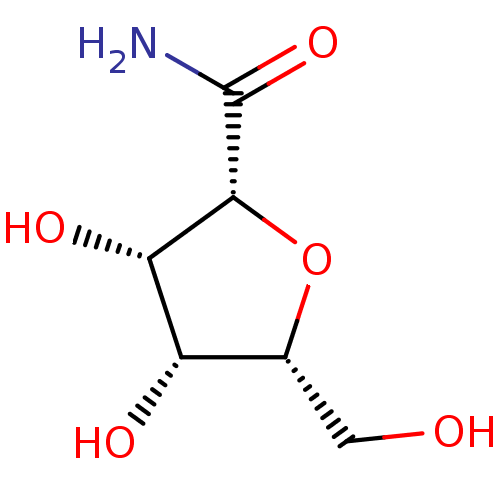
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Goeminne, A; McNaughton, M; Bal, G; Surpateanu, G; Van Der Veken, P; De Prol, S; Versées, W; Steyaert, J; Haemers, A; Augustyns, K Synthesis and biochemical evaluation of guanidino-alkyl-ribitol derivatives as nucleoside hydrolase inhibitors. Eur J Med Chem43:315-26 (2008) [PubMed] Article
Goeminne, A; McNaughton, M; Bal, G; Surpateanu, G; Van Der Veken, P; De Prol, S; Versées, W; Steyaert, J; Haemers, A; Augustyns, K Synthesis and biochemical evaluation of guanidino-alkyl-ribitol derivatives as nucleoside hydrolase inhibitors. Eur J Med Chem43:315-26 (2008) [PubMed] Article