| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Macrophage-stimulating protein receptor |
|---|
| Ligand | BDBM50242740 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_531097 (CHEMBL976905) |
|---|
| EC50 | >2000±n/a nM |
|---|
| Citation |  McDermott, U; Sharma, SV; Dowell, L; Greninger, P; Montagut, C; Lamb, J; Archibald, H; Raudales, R; Tam, A; Lee, D; Rothenberg, SM; Supko, JG; Sordella, R; Ulkus, LE; Iafrate, AJ; Maheswaran, S; Njauw, CN; Tsao, H; Drew, L; Hanke, JH; Ma, XJ; Erlander, MG; Gray, NS; Haber, DA; Settleman, J Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci U S A104:19936-41 (2007) [PubMed] Article McDermott, U; Sharma, SV; Dowell, L; Greninger, P; Montagut, C; Lamb, J; Archibald, H; Raudales, R; Tam, A; Lee, D; Rothenberg, SM; Supko, JG; Sordella, R; Ulkus, LE; Iafrate, AJ; Maheswaran, S; Njauw, CN; Tsao, H; Drew, L; Hanke, JH; Ma, XJ; Erlander, MG; Gray, NS; Haber, DA; Settleman, J Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci U S A104:19936-41 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Macrophage-stimulating protein receptor |
|---|
| Name: | Macrophage-stimulating protein receptor |
|---|
| Synonyms: | 2.7.10.1 | CD_antigen=CD136 | CDw136 | MSP receptor | MST1R | Macrophage-stimulating protein receptor (MST1R) | Macrophage-stimulating protein receptor alpha chain | Macrophage-stimulating protein receptor beta chain | PTK8 | Protein-tyrosine kinase 8 | RON | RON_HUMAN | Tyrosine kinase receptor ron | p185-Ron |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 152270.76 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q04912 |
|---|
| Residue: | 1400 |
|---|
| Sequence: | MELLPPLPQSFLLLLLLPAKPAAGEDWQCPRTPYAASRDFDVKYVVPSFSAGGLVQAMVT
YEGDRNESAVFVAIRNRLHVLGPDLKSVQSLATGPAGDPGCQTCAACGPGPHGPPGDTDT
KVLVLDPALPALVSCGSSLQGRCFLHDLEPQGTAVHLAAPACLFSAHHNRPDDCPDCVAS
PLGTRVTVVEQGQASYFYVASSLDAAVAASFSPRSVSIRRLKADASGFAPGFVALSVLPK
HLVSYSIEYVHSFHTGAFVYFLTVQPASVTDDPSALHTRLARLSATEPELGDYRELVLDC
RFAPKRRRRGAPEGGQPYPVLRVAHSAPVGAQLATELSIAEGQEVLFGVFVTGKDGGPGV
GPNSVVCAFPIDLLDTLIDEGVERCCESPVHPGLRRGLDFFQSPSFCPNPPGLEALSPNT
SCRHFPLLVSSSFSRVDLFNGLLGPVQVTALYVTRLDNVTVAHMGTMDGRILQVELVRSL
NYLLYVSNFSLGDSGQPVQRDVSRLGDHLLFASGDQVFQVPIQGPGCRHFLTCGRCLRAW
HFMGCGWCGNMCGQQKECPGSWQQDHCPPKLTEFHPHSGPLRGSTRLTLCGSNFYLHPSG
LVPEGTHQVTVGQSPCRPLPKDSSKLRPVPRKDFVEEFECELEPLGTQAVGPTNVSLTVT
NMPPGKHFRVDGTSVLRGFSFMEPVLIAVQPLFGPRAGGTCLTLEGQSLSVGTSRAVLVN
GTECLLARVSEGQLLCATPPGATVASVPLSLQVGGAQVPGSWTFQYREDPVVLSISPNCG
YINSHITICGQHLTSAWHLVLSFHDGLRAVESRCERQLPEQQLCRLPEYVVRDPQGWVAG
NLSARGDGAAGFTLPGFRFLPPPHPPSANLVPLKPEEHAIKFEYIGLGAVADCVGINVTV
GGESCQHEFRGDMVVCPLPPSLQLGQDGAPLQVCVDGECHILGRVVRPGPDGVPQSTLLG
ILLPLLLLVAALATALVFSYWWRRKQLVLPPNLNDLASLDQTAGATPLPILYSGSDYRSG
LALPAIDGLDSTTCVHGASFSDSEDESCVPLLRKESIQLRDLDSALLAEVKDVLIPHERV
VTHSDRVIGKGHFGVVYHGEYIDQAQNRIQCAIKSLSRITEMQQVEAFLREGLLMRGLNH
PNVLALIGIMLPPEGLPHVLLPYMCHGDLLQFIRSPQRNPTVKDLISFGLQVARGMEYLA
EQKFVHRDLAARNCMLDESFTVKVADFGLARDILDREYYSVQQHRHARLPVKWMALESLQ
TYRFTTKSDVWSFGVLLWELLTRGAPPYRHIDPFDLTHFLAQGRRLPQPEYCPDSLYQVM
QQCWEADPAVRPTFRVLVGEVEQIVSALLGDHYVQLPATYMNLGPSTSHEMNVRPEQPQF
SPMPGNVRRPRPLSEPPRPT
|
|
|
|---|
| BDBM50242740 |
|---|
| n/a |
|---|
| Name | BDBM50242740 |
|---|
| Synonyms: | CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-dihydropyrimido[4,5-d]pyrimidin-3(4H)-yl)-4-methylphenyl)-3-(trifluoromethyl)benzamide | Type II inhibitor, 14 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H19F3N6O2 |
|---|
| Mol. Mass. | 456.4205 |
|---|
| SMILES | CN1C(=O)N(Cc2cnc(N)nc12)c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C |
|---|
| Structure | 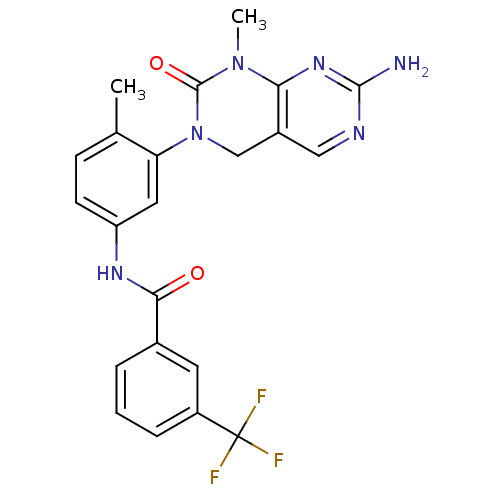
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 McDermott, U; Sharma, SV; Dowell, L; Greninger, P; Montagut, C; Lamb, J; Archibald, H; Raudales, R; Tam, A; Lee, D; Rothenberg, SM; Supko, JG; Sordella, R; Ulkus, LE; Iafrate, AJ; Maheswaran, S; Njauw, CN; Tsao, H; Drew, L; Hanke, JH; Ma, XJ; Erlander, MG; Gray, NS; Haber, DA; Settleman, J Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci U S A104:19936-41 (2007) [PubMed] Article
McDermott, U; Sharma, SV; Dowell, L; Greninger, P; Montagut, C; Lamb, J; Archibald, H; Raudales, R; Tam, A; Lee, D; Rothenberg, SM; Supko, JG; Sordella, R; Ulkus, LE; Iafrate, AJ; Maheswaran, S; Njauw, CN; Tsao, H; Drew, L; Hanke, JH; Ma, XJ; Erlander, MG; Gray, NS; Haber, DA; Settleman, J Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci U S A104:19936-41 (2007) [PubMed] Article