| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Aurora kinase B |
|---|
| Ligand | BDBM26300 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_501683 (CHEMBL981431) |
|---|
| IC50 | 17±n/a nM |
|---|
| Citation |  Pollard, JR; Mortimore, M Discovery and development of aurora kinase inhibitors as anticancer agents. J Med Chem52:2629-51 (2009) [PubMed] Article Pollard, JR; Mortimore, M Discovery and development of aurora kinase inhibitors as anticancer agents. J Med Chem52:2629-51 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Aurora kinase B |
|---|
| Name: | Aurora kinase B |
|---|
| Synonyms: | AIK2 | AIM-1 | AIM1 | AIRK2 | ARK2 | AURKB | AURKB_HUMAN | Aurora B kinase (aurB) | Aurora B-INCENP | Aurora kinase 2 | Aurora kinase B (AURKB) | Aurora-related kinase 2 | STK-1 | STK1 | STK12 | STK5 | Serine/threonine-protein kinase aurora B |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 39327.72 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q96GD4 |
|---|
| Residue: | 344 |
|---|
| Sequence: | MAQKENSYPWPYGRQTAPSGLSTLPQRVLRKEPVTPSALVLMSRSNVQPTAAPGQKVMEN
SSGTPDILTRHFTIDDFEIGRPLGKGKFGNVYLAREKKSHFIVALKVLFKSQIEKEGVEH
QLRREIEIQAHLHHPNILRLYNYFYDRRRIYLILEYAPRGELYKELQKSCTFDEQRTATI
MEELADALMYCHGKKVIHRDIKPENLLLGLKGELKIADFGWSVHAPSLRRKTMCGTLDYL
PPEMIEGRMHNEKVDLWCIGVLCYELLVGNPPFESASHNETYRRIVKVDLKFPASVPMGA
QDLISKLLRHNPSERLPLAQVSAHPWVRANSRRVLPPSALQSVA
|
|
|
|---|
| BDBM26300 |
|---|
| n/a |
|---|
| Name | BDBM26300 |
|---|
| Synonyms: | 2-{3-[(7-{3-[ethyl(2-hydroxyethyl)amino]propoxy}quinazolin-4-yl)amino]-1H-pyrazol-5-yl}-N-(3-fluorophenyl)acetamide | CHEMBL215152 | cid_16007391 | pyrazoloquinazoline deriv., 34 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H30FN7O3 |
|---|
| Mol. Mass. | 507.5599 |
|---|
| SMILES | CCN(CCO)CCCOc1ccc2c(Nc3cc(CC(=O)Nc4cccc(F)c4)n[nH]3)ncnc2c1 |
|---|
| Structure | 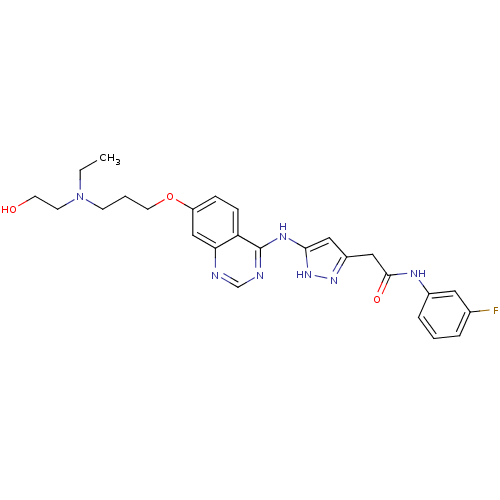
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Pollard, JR; Mortimore, M Discovery and development of aurora kinase inhibitors as anticancer agents. J Med Chem52:2629-51 (2009) [PubMed] Article
Pollard, JR; Mortimore, M Discovery and development of aurora kinase inhibitors as anticancer agents. J Med Chem52:2629-51 (2009) [PubMed] Article