| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A2b |
|---|
| Ligand | BDBM50308501 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_613348 (CHEMBL1074285) |
|---|
| Ki | 391±n/a nM |
|---|
| Citation |  Scheiff, AB; Yerande, SG; El-Tayeb, A; Li, W; Inamdar, GS; Vasu, KK; Sudarsanam, V; Müller, CE 2-Amino-5-benzoyl-4-phenylthiazoles: Development of potent and selective adenosine A1 receptor antagonists. Bioorg Med Chem18:2195-203 (2010) [PubMed] Article Scheiff, AB; Yerande, SG; El-Tayeb, A; Li, W; Inamdar, GS; Vasu, KK; Sudarsanam, V; Müller, CE 2-Amino-5-benzoyl-4-phenylthiazoles: Development of potent and selective adenosine A1 receptor antagonists. Bioorg Med Chem18:2195-203 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A2b |
|---|
| Name: | Adenosine receptor A2b |
|---|
| Synonyms: | AA2BR_HUMAN | ADENOSINE A2B | ADORA2B | Adenosine receptor A2B (A2B) | Adenosine receptors A2b | Adenosine receptors; A2a & A2b |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 36341.22 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 332 |
|---|
| Sequence: | MLLETQDALYVALELVIAALSVAGNVLVCAAVGTANTLQTPTNYFLVSLAAADVAVGLFA
IPFAITISLGFCTDFYGCLFLACFVLVLTQSSIFSLLAVAVDRYLAICVPLRYKSLVTGT
RARGVIAVLWVLAFGIGLTPFLGWNSKDSATNNCTEPWDGTTNESCCLVKCLFENVVPMS
YMVYFNFFGCVLPPLLIMLVIYIKIFLVACRQLQRTELMDHSRTTLQREIHAAKSLAMIV
GIFALCWLPVHAVNCVTLFQPAQGKNKPKWAMNMAILLSHANSVVNPIVYAYRNRDFRYT
FHKIISRYLLCQADVKSGNGQAGVQPALGVGL
|
|
|
|---|
| BDBM50308501 |
|---|
| n/a |
|---|
| Name | BDBM50308501 |
|---|
| Synonyms: | CHEMBL603129 | Ethyl 5-benzoyl-4-phenylthiazol-2-ylcarbamate | cid_7315331 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H16N2O3S |
|---|
| Mol. Mass. | 352.407 |
|---|
| SMILES | CCOC(=O)Nc1nc(c(s1)C(=O)c1ccccc1)-c1ccccc1 |
|---|
| Structure | 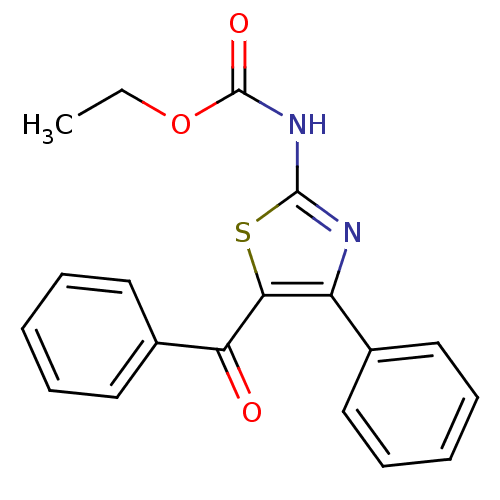
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Scheiff, AB; Yerande, SG; El-Tayeb, A; Li, W; Inamdar, GS; Vasu, KK; Sudarsanam, V; Müller, CE 2-Amino-5-benzoyl-4-phenylthiazoles: Development of potent and selective adenosine A1 receptor antagonists. Bioorg Med Chem18:2195-203 (2010) [PubMed] Article
Scheiff, AB; Yerande, SG; El-Tayeb, A; Li, W; Inamdar, GS; Vasu, KK; Sudarsanam, V; Müller, CE 2-Amino-5-benzoyl-4-phenylthiazoles: Development of potent and selective adenosine A1 receptor antagonists. Bioorg Med Chem18:2195-203 (2010) [PubMed] Article