| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Albumin |
|---|
| Ligand | BDBM50335522 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_705623 (CHEMBL1661507) |
|---|
| Kd | 12000±n/a nM |
|---|
| Citation |  Banères-Roquet, F; Gualtieri, M; Villain-Guillot, P; Pugnière, M; Leonetti, JP Use of a surface plasmon resonance method to investigate antibiotic and plasma protein interactions. Antimicrob Agents Chemother53:1528-31 (2009) [PubMed] Article Banères-Roquet, F; Gualtieri, M; Villain-Guillot, P; Pugnière, M; Leonetti, JP Use of a surface plasmon resonance method to investigate antibiotic and plasma protein interactions. Antimicrob Agents Chemother53:1528-31 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Albumin |
|---|
| Name: | Albumin |
|---|
| Synonyms: | ALB | ALBU_HUMAN | Serum albumin |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 69362.94 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1509401 |
|---|
| Residue: | 609 |
|---|
| Sequence: | MKWVTFISLLFLFSSAYSRGVFRRDAHKSEVAHRFKDLGEENFKALVLIAFAQYLQQCPF
EDHVKLVNEVTEFAKTCVADESAENCDKSLHTLFGDKLCTVATLRETYGEMADCCAKQEP
ERNECFLQHKDDNPNLPRLVRPEVDVMCTAFHDNEETFLKKYLYEIARRHPYFYAPELLF
FAKRYKAAFTECCQAADKAACLLPKLDELRDEGKASSAKQRLKCASLQKFGERAFKAWAV
ARLSQRFPKAEFAEVSKLVTDLTKVHTECCHGDLLECADDRADLAKYICENQDSISSKLK
ECCEKPLLEKSHCIAEVENDEMPADLPSLAADFVESKDVCKNYAEAKDVFLGMFLYEYAR
RHPDYSVVLLLRLAKTYETTLEKCCAAADPHECYAKVFDEFKPLVEEPQNLIKQNCELFE
QLGEYKFQNALLVRYTKKVPQVSTPTLVEVSRNLGKVGSKCCKHPEAKRMPCAEDYLSVV
LNQLCVLHEKTPVSDRVTKCCTESLVNRRPCFSALEVDETYVPKEFNAETFTFHADICTL
SEKERQIKKQTALVELVKHKPKATKEQLKAVMDDFAAFVEKCCKADDKETCFAEEGKKLV
AASQAALGL
|
|
|
|---|
| BDBM50335522 |
|---|
| n/a |
|---|
| Name | BDBM50335522 |
|---|
| Synonyms: | (2S,4R)-N-((1R,2R)-2-hydroxy-1-((2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(methylthio)-tetrahydro-2H-pyran-2-yl)propyl)-1-methyl-4-propylpyrrolidine-2-carboxamide | (2S,4R)-N-((1S)-2-hydroxy-1-((2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(methylthio)-tetrahydro-2H-pyran-2-yl)propyl)-1-methyl-4-propylpyrrolidine-2-carboxamide | 1-Methyl-4-propyl-pyrrolidine-2-carboxylic acid [2-hydroxy-1-(3,4,5-trihydroxy-6-methylsulfanyl-tetrahydro-pyran-2-yl)-propyl]-amide | CHEMBL1447 | Cillimycin | Frademicina | LINCOMYCIN | Lincocin | Lincomycin hydrochloride | lincomycin A |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H34N2O6S |
|---|
| Mol. Mass. | 406.537 |
|---|
| SMILES | CCC[C@@H]1C[C@H](N(C)C1)C(=O)N[C@H]([C@@H](C)O)[C@H]1O[C@H](SC)[C@H](O)[C@@H](O)[C@H]1O |r| |
|---|
| Structure | 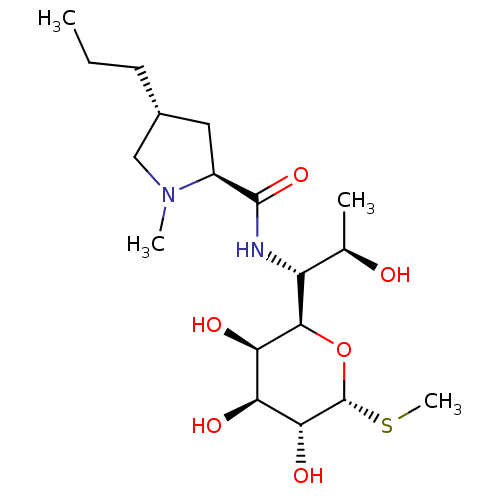
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Banères-Roquet, F; Gualtieri, M; Villain-Guillot, P; Pugnière, M; Leonetti, JP Use of a surface plasmon resonance method to investigate antibiotic and plasma protein interactions. Antimicrob Agents Chemother53:1528-31 (2009) [PubMed] Article
Banères-Roquet, F; Gualtieri, M; Villain-Guillot, P; Pugnière, M; Leonetti, JP Use of a surface plasmon resonance method to investigate antibiotic and plasma protein interactions. Antimicrob Agents Chemother53:1528-31 (2009) [PubMed] Article