| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Vascular endothelial growth factor receptor 2 |
|---|
| Ligand | BDBM50338873 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_727273 (CHEMBL1687367) |
|---|
| IC50 | 9.42±n/a nM |
|---|
| Citation |  Kim, MH; Kim, M; Yu, H; Kim, H; Yoo, KH; Sim, T; Hah, JM Structure based design and syntheses of amino-1H-pyrazole amide derivatives as selective Raf kinase inhibitors in melanoma cells. Bioorg Med Chem19:1915-23 (2011) [PubMed] Article Kim, MH; Kim, M; Yu, H; Kim, H; Yoo, KH; Sim, T; Hah, JM Structure based design and syntheses of amino-1H-pyrazole amide derivatives as selective Raf kinase inhibitors in melanoma cells. Bioorg Med Chem19:1915-23 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Vascular endothelial growth factor receptor 2 |
|---|
| Name: | Vascular endothelial growth factor receptor 2 |
|---|
| Synonyms: | CD_antigen=CD309 | FLK1 | Fetal liver kinase 1 (FLK-1) | Flk-1/KDR | KDR | Kinase Insert Domain Receptor | Protein-tyrosine kinase receptor Flk-1 | VEGFR kinase (KDR) | VEGFR-2 | VEGFR-2 (KDR) | VEGFR2 | VGFR2_HUMAN | Vascular Endothelial Growth Factor Receptor Kinase 2 | Vascular endothelial growth factor receptor (VEGFR-2) | Vascular endothelial growth factor receptor 2 (KDR) | Vascular endothelial growth factor receptor 2 (VEGFR-2) | Vascular endothelial growth factor receptor 2 (VEGFR2) | Vascular endothelial growth factor receptor 2 precursor (VEGFR-2) | Vascular endothelial growth factor receptor-2 (VEGFR-2) |
|---|
| Type: | Receptor Tyrosine Kinase |
|---|
| Mol. Mass.: | 151510.97 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P35968 |
|---|
| Residue: | 1356 |
|---|
| Sequence: | MQSKVLLAVALWLCVETRAASVGLPSVSLDLPRLSIQKDILTIKANTTLQITCRGQRDLD
WLWPNNQSGSEQRVEVTECSDGLFCKTLTIPKVIGNDTGAYKCFYRETDLASVIYVYVQD
YRSPFIASVSDQHGVVYITENKNKTVVIPCLGSISNLNVSLCARYPEKRFVPDGNRISWD
SKKGFTIPSYMISYAGMVFCEAKINDESYQSIMYIVVVVGYRIYDVVLSPSHGIELSVGE
KLVLNCTARTELNVGIDFNWEYPSSKHQHKKLVNRDLKTQSGSEMKKFLSTLTIDGVTRS
DQGLYTCAASSGLMTKKNSTFVRVHEKPFVAFGSGMESLVEATVGERVRIPAKYLGYPPP
EIKWYKNGIPLESNHTIKAGHVLTIMEVSERDTGNYTVILTNPISKEKQSHVVSLVVYVP
PQIGEKSLISPVDSYQYGTTQTLTCTVYAIPPPHHIHWYWQLEEECANEPSQAVSVTNPY
PCEEWRSVEDFQGGNKIEVNKNQFALIEGKNKTVSTLVIQAANVSALYKCEAVNKVGRGE
RVISFHVTRGPEITLQPDMQPTEQESVSLWCTADRSTFENLTWYKLGPQPLPIHVGELPT
PVCKNLDTLWKLNATMFSNSTNDILIMELKNASLQDQGDYVCLAQDRKTKKRHCVVRQLT
VLERVAPTITGNLENQTTSIGESIEVSCTASGNPPPQIMWFKDNETLVEDSGIVLKDGNR
NLTIRRVRKEDEGLYTCQACSVLGCAKVEAFFIIEGAQEKTNLEIIILVGTAVIAMFFWL
LLVIILRTVKRANGGELKTGYLSIVMDPDELPLDEHCERLPYDASKWEFPRDRLKLGKPL
GRGAFGQVIEADAFGIDKTATCRTVAVKMLKEGATHSEHRALMSELKILIHIGHHLNVVN
LLGACTKPGGPLMVIVEFCKFGNLSTYLRSKRNEFVPYKTKGARFRQGKDYVGAIPVDLK
RRLDSITSSQSSASSGFVEEKSLSDVEEEEAPEDLYKDFLTLEHLICYSFQVAKGMEFLA
SRKCIHRDLAARNILLSEKNVVKICDFGLARDIYKDPDYVRKGDARLPLKWMAPETIFDR
VYTIQSDVWSFGVLLWEIFSLGASPYPGVKIDEEFCRRLKEGTRMRAPDYTTPEMYQTML
DCWHGEPSQRPTFSELVEHLGNLLQANAQQDGKDYIVLPISETLSMEEDSGLSLPTSPVS
CMEEEEVCDPKFHYDNTAGISQYLQNSKRKSRPVSVKTFEDIPLEEPEVKVIPDDNQTDS
GMVLASEELKTLEDRTKLSPSFGGMVPSKSRESVASEGSNQTSGYQSGYHSDDTDTTVYS
SEEAELLKLIEIGVQTGSTAQILQPDSGTTLSSPPV
|
|
|
|---|
| BDBM50338873 |
|---|
| n/a |
|---|
| Name | BDBM50338873 |
|---|
| Synonyms: | CHEMBL1684800 | N-(5-Amino-1-(4-methoxybenzyl)-1H-pyrazol-4-yl)-5-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)-2-methylbenzamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C27H24ClF3N6O3 |
|---|
| Mol. Mass. | 572.966 |
|---|
| SMILES | COc1ccc(Cn2ncc(NC(=O)c3cc(NC(=O)Nc4ccc(Cl)c(c4)C(F)(F)F)ccc3C)c2N)cc1 |
|---|
| Structure | 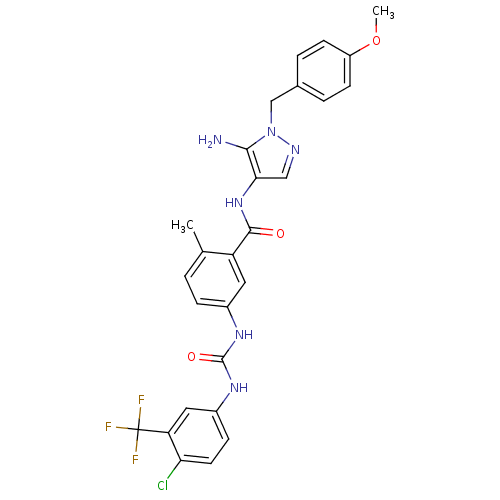
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kim, MH; Kim, M; Yu, H; Kim, H; Yoo, KH; Sim, T; Hah, JM Structure based design and syntheses of amino-1H-pyrazole amide derivatives as selective Raf kinase inhibitors in melanoma cells. Bioorg Med Chem19:1915-23 (2011) [PubMed] Article
Kim, MH; Kim, M; Yu, H; Kim, H; Yoo, KH; Sim, T; Hah, JM Structure based design and syntheses of amino-1H-pyrazole amide derivatives as selective Raf kinase inhibitors in melanoma cells. Bioorg Med Chem19:1915-23 (2011) [PubMed] Article