| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Neutral ceramidase |
|---|
| Ligand | BDBM50392471 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_852173 (CHEMBL2157594) |
|---|
| IC50 | 54000±n/a nM |
|---|
| Citation |  Bhabak, KP; Arenz, C Novel amide- and sulfonamide-based aromatic ethanolamines: effects of various substituents on the inhibition of acid and neutral ceramidases. Bioorg Med Chem20:6162-70 (2012) [PubMed] Article Bhabak, KP; Arenz, C Novel amide- and sulfonamide-based aromatic ethanolamines: effects of various substituents on the inhibition of acid and neutral ceramidases. Bioorg Med Chem20:6162-70 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Neutral ceramidase |
|---|
| Name: | Neutral ceramidase |
|---|
| Synonyms: | ASAH2 | ASAH2_HUMAN | Acylsphingosine deacylase 2 | BCDase | HNAC1 | LCDase | N-CDase | N-acylsphingosine amidohydrolase 2 | NCDase | Neutral ceramidase soluble form | Non-lysosomal ceramidase | hCD |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 85523.37 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_947546 |
|---|
| Residue: | 780 |
|---|
| Sequence: | MAKRTFSNLETFLIFLLVMMSAITVALLSLLFITSGTIENHKDLGGHFFSTTQSPPATQG
STAAQRSTATQHSTATQSSTATQTSPVPLTPESPLFQNFSGYHIGVGRADCTGQVADINL
MGYGKSGQNAQGILTRLYSRAFIMAEPDGSNRTVFVSIDIGMVSQRLRLEVLNRLQSKYG
SLYRRDNVILSGTHTHSGPAGYFQYTVFVIASEGFSNQTFQHMVTGILKSIDIAHTNMKP
GKIFINKGNVDGVQINRSPYSYLQNPQSERARYSSNTDKEMIVLKMVDLNGDDLGLISWF
AIHPVSMNNSNHLVNSDNVGYASYLLEQEKNKGYLPGQGPFVAAFASSNLGDVSPNILGP
RCINTGESCDNANSTCPIGGPSMCIAKGPGQDMFDSTQIIGRAMYQRAKELYASASQEVT
GPLASAHQWVDMTDVTVWLNSTHASKTCKPALGYSFAAGTIDGVGGLNFTQGKTEGDPFW
DTIRDQILGKPSEEIKECHKPKPILLHTGELSKPHPWHPDIVDVQIITLGSLAITAIPGE
FTTMSGRRLREAVQAEFASHGMQNMTVVISGLCNVYTHYITTYEEYQAQRYEAASTIYGP
HTLSAYIQLFRNLAKAIATDTVANLSRGPEPPFFKQLIVPLIPSIVDRAPKGRTFGDVLQ
PAKPEYRVGEVAEVIFVGANPKNSVQNQTHQTFLTVEKYEATSTSWQIVCNDASWETRFY
WHKGLLGLSNATVEWHIPDTAQPGIYRIRYFGHNRKQDILKPAVILSFEGTSPAFEVVTI
|
|
|
|---|
| BDBM50392471 |
|---|
| n/a |
|---|
| Name | BDBM50392471 |
|---|
| Synonyms: | CHEMBL2152040 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H36N2O6S |
|---|
| Mol. Mass. | 444.585 |
|---|
| SMILES | CCCCCCCCCCCCS(=O)(=O)N[C@H](CO)[C@H](O)c1ccc(cc1)[N+]([O-])=O |r| |
|---|
| Structure | 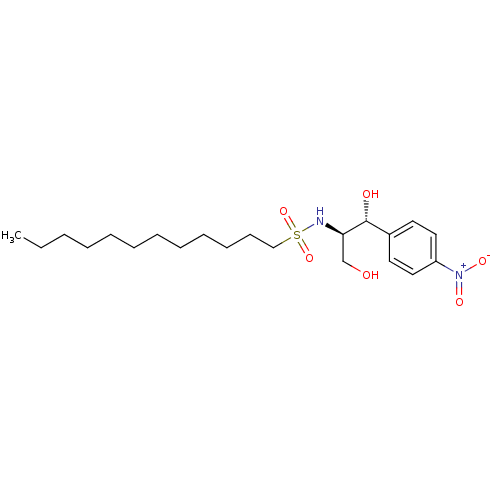
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bhabak, KP; Arenz, C Novel amide- and sulfonamide-based aromatic ethanolamines: effects of various substituents on the inhibition of acid and neutral ceramidases. Bioorg Med Chem20:6162-70 (2012) [PubMed] Article
Bhabak, KP; Arenz, C Novel amide- and sulfonamide-based aromatic ethanolamines: effects of various substituents on the inhibition of acid and neutral ceramidases. Bioorg Med Chem20:6162-70 (2012) [PubMed] Article