| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cannabinoid receptor 1 |
|---|
| Ligand | BDBM50420876 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_840018 (CHEMBL2090508) |
|---|
| Ki | >10000±n/a nM |
|---|
| Citation |  Pasquini, S; Mugnaini, C; Ligresti, A; Tafi, A; Brogi, S; Falciani, C; Pedani, V; Pesco, N; Guida, F; Luongo, L; Varani, K; Borea, PA; Maione, S; Di Marzo, V; Corelli, F Design, synthesis, and pharmacological characterization of indol-3-ylacetamides, indol-3-yloxoacetamides, and indol-3-ylcarboxamides: potent and selective CB2 cannabinoid receptor inverse agonists. J Med Chem55:5391-402 (2012) [PubMed] Article Pasquini, S; Mugnaini, C; Ligresti, A; Tafi, A; Brogi, S; Falciani, C; Pedani, V; Pesco, N; Guida, F; Luongo, L; Varani, K; Borea, PA; Maione, S; Di Marzo, V; Corelli, F Design, synthesis, and pharmacological characterization of indol-3-ylacetamides, indol-3-yloxoacetamides, and indol-3-ylcarboxamides: potent and selective CB2 cannabinoid receptor inverse agonists. J Med Chem55:5391-402 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cannabinoid receptor 1 |
|---|
| Name: | Cannabinoid receptor 1 |
|---|
| Synonyms: | CANN6 | CANNABINOID CB1 | CB-R | CB1 | CNR | CNR1 | CNR1_HUMAN | Cannabinoid CB1 receptor | Cannabinoid receptor | Cannabinoid receptor 1 (CB1) | Cannabinoid receptor 1 (brain) |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 52868.96 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P21554 |
|---|
| Residue: | 472 |
|---|
| Sequence: | MKSILDGLADTTFRTITTDLLYVGSNDIQYEDIKGDMASKLGYFPQKFPLTSFRGSPFQE
KMTAGDNPQLVPADQVNITEFYNKSLSSFKENEENIQCGENFMDIECFMVLNPSQQLAIA
VLSLTLGTFTVLENLLVLCVILHSRSLRCRPSYHFIGSLAVADLLGSVIFVYSFIDFHVF
HRKDSRNVFLFKLGGVTASFTASVGSLFLTAIDRYISIHRPLAYKRIVTRPKAVVAFCLM
WTIAIVIAVLPLLGWNCEKLQSVCSDIFPHIDETYLMFWIGVTSVLLLFIVYAYMYILWK
AHSHAVRMIQRGTQKSIIIHTSEDGKVQVTRPDQARMDIRLAKTLVLILVVLIICWGPLL
AIMVYDVFGKMNKLIKTVFAFCSMLCLLNSTVNPIIYALRSKDLRHAFRSMFPSCEGTAQ
PLDNSMGDSDCLHKHANNAASVHRAAESCIKSTVKIAKVTMSVSTDTSAEAL
|
|
|
|---|
| BDBM50420876 |
|---|
| n/a |
|---|
| Name | BDBM50420876 |
|---|
| Synonyms: | CHEMBL2087100 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H36N2O2 |
|---|
| Mol. Mass. | 468.6297 |
|---|
| SMILES | CCCCCn1cc(C(=O)C(=O)NC23CC4CC(CC(C4)C2)C3)c2cc(ccc12)-c1ccccc1 |TLB:12:13:16:20.19.18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13| |
|---|
| Structure | 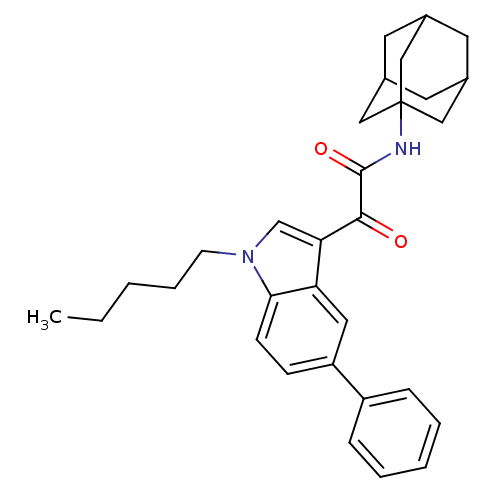
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Pasquini, S; Mugnaini, C; Ligresti, A; Tafi, A; Brogi, S; Falciani, C; Pedani, V; Pesco, N; Guida, F; Luongo, L; Varani, K; Borea, PA; Maione, S; Di Marzo, V; Corelli, F Design, synthesis, and pharmacological characterization of indol-3-ylacetamides, indol-3-yloxoacetamides, and indol-3-ylcarboxamides: potent and selective CB2 cannabinoid receptor inverse agonists. J Med Chem55:5391-402 (2012) [PubMed] Article
Pasquini, S; Mugnaini, C; Ligresti, A; Tafi, A; Brogi, S; Falciani, C; Pedani, V; Pesco, N; Guida, F; Luongo, L; Varani, K; Borea, PA; Maione, S; Di Marzo, V; Corelli, F Design, synthesis, and pharmacological characterization of indol-3-ylacetamides, indol-3-yloxoacetamides, and indol-3-ylcarboxamides: potent and selective CB2 cannabinoid receptor inverse agonists. J Med Chem55:5391-402 (2012) [PubMed] Article