| Citation |  Fugel, W; Oberholzer, AE; Gschloessl, B; Dzikowski, R; Pressburger, N; Preu, L; Pearl, LH; Baratte, B; Ratin, M; Okun, I; Doerig, C; Kruggel, S; Lemcke, T; Meijer, L; Kunick, C 3,6-Diamino-4-(2-halophenyl)-2-benzoylthieno[2,3-b]pyridine-5-carbonitriles are selective inhibitors of Plasmodium falciparum glycogen synthase kinase-3. J Med Chem56:264-75 (2013) [PubMed] Article Fugel, W; Oberholzer, AE; Gschloessl, B; Dzikowski, R; Pressburger, N; Preu, L; Pearl, LH; Baratte, B; Ratin, M; Okun, I; Doerig, C; Kruggel, S; Lemcke, T; Meijer, L; Kunick, C 3,6-Diamino-4-(2-halophenyl)-2-benzoylthieno[2,3-b]pyridine-5-carbonitriles are selective inhibitors of Plasmodium falciparum glycogen synthase kinase-3. J Med Chem56:264-75 (2013) [PubMed] Article |
|---|
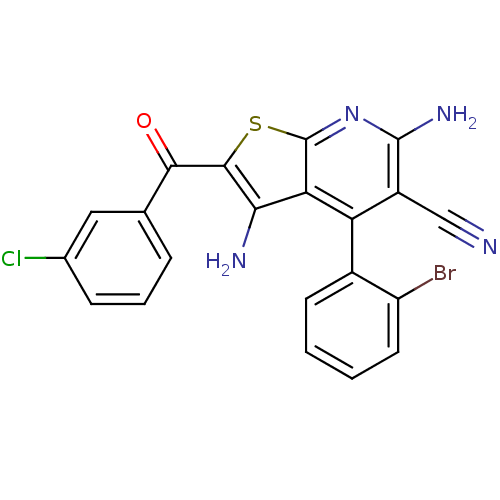
| SMILES | Nc1c(sc2nc(N)c(C#N)c(-c3ccccc3Br)c12)C(=O)c1cccc(Cl)c1 |(50.21,-49.5,;49.73,-50.97,;50.65,-52.22,;49.74,-53.48,;48.26,-53,;46.93,-53.77,;45.59,-53,;44.26,-53.77,;45.6,-51.45,;44.26,-50.67,;42.93,-49.9,;46.92,-50.68,;46.91,-49.15,;48.25,-48.38,;48.24,-46.84,;46.91,-46.07,;45.57,-46.85,;45.58,-48.39,;44.25,-49.17,;48.26,-51.45,;52.19,-52.22,;52.96,-50.89,;52.96,-53.56,;52.18,-54.89,;52.95,-56.22,;54.49,-56.22,;55.26,-54.88,;56.8,-54.87,;54.49,-53.55,)| |
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Fugel, W; Oberholzer, AE; Gschloessl, B; Dzikowski, R; Pressburger, N; Preu, L; Pearl, LH; Baratte, B; Ratin, M; Okun, I; Doerig, C; Kruggel, S; Lemcke, T; Meijer, L; Kunick, C 3,6-Diamino-4-(2-halophenyl)-2-benzoylthieno[2,3-b]pyridine-5-carbonitriles are selective inhibitors of Plasmodium falciparum glycogen synthase kinase-3. J Med Chem56:264-75 (2013) [PubMed] Article
Fugel, W; Oberholzer, AE; Gschloessl, B; Dzikowski, R; Pressburger, N; Preu, L; Pearl, LH; Baratte, B; Ratin, M; Okun, I; Doerig, C; Kruggel, S; Lemcke, T; Meijer, L; Kunick, C 3,6-Diamino-4-(2-halophenyl)-2-benzoylthieno[2,3-b]pyridine-5-carbonitriles are selective inhibitors of Plasmodium falciparum glycogen synthase kinase-3. J Med Chem56:264-75 (2013) [PubMed] Article