| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acrosin |
|---|
| Ligand | BDBM50424443 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_936791 (CHEMBL2320183) |
|---|
| IC50 | 80000±n/a nM |
|---|
| Citation |  Chen, Q; Tian, W; Han, G; Qi, J; Zheng, C; Zhou, Y; Ding, L; Zhao, J; Zhu, J; Lv, J; Sheng, C Design and synthesis of novel benzoheterocyclic derivatives as human acrosin inhibitors by scaffold hopping. Eur J Med Chem59:176-82 (2013) [PubMed] Article Chen, Q; Tian, W; Han, G; Qi, J; Zheng, C; Zhou, Y; Ding, L; Zhao, J; Zhu, J; Lv, J; Sheng, C Design and synthesis of novel benzoheterocyclic derivatives as human acrosin inhibitors by scaffold hopping. Eur J Med Chem59:176-82 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acrosin |
|---|
| Name: | Acrosin |
|---|
| Synonyms: | ACR | ACRO_HUMAN | ACRS |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 45865.48 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1365286 |
|---|
| Residue: | 421 |
|---|
| Sequence: | MVEMLPTAILLVLAVSVVAKDNATCDGPCGLRFRQNPQGGVRIVGGKAAQHGAWPWMVSL
QIFTYNSHRYHTCGGSLLNSRWVLTAAHCFVGKNNVHDWRLVFGAKEITYGNNKPVKAPL
QERYVEKIIIHEKYNSATEGNDIALVEITPPISCGRFIGPGCLPHFKAGLPRGSQSCWVA
GWGYIEEKAPRPSSILMEARVDLIDLDLCNSTQWYNGRVQPTNVCAGYPVGKIDTCQGDS
GGPLMCKDSKESAYVVVGITSWGVGCARAKRPGIYTATWPYLNWIASKIGSNALRMIQSA
TPPPPTTRPPPIRPPFSHPISAHLPWYFQPPPRPLPPRPPAAQPRPPPSPPPPPPPPASP
LPPPPPPPPPTPSSTTKLPQGLSFAKRLQQLIEVLKGKTYSDGKNHYDMETTELPELTST
S
|
|
|
|---|
| BDBM50424443 |
|---|
| n/a |
|---|
| Name | BDBM50424443 |
|---|
| Synonyms: | CHEMBL2316070 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H18FN5O4S |
|---|
| Mol. Mass. | 407.419 |
|---|
| SMILES | OCCNC(=O)Nc1nc2ccc(cc2[nH]1)S(=O)(=O)NCc1ccccc1F |
|---|
| Structure | 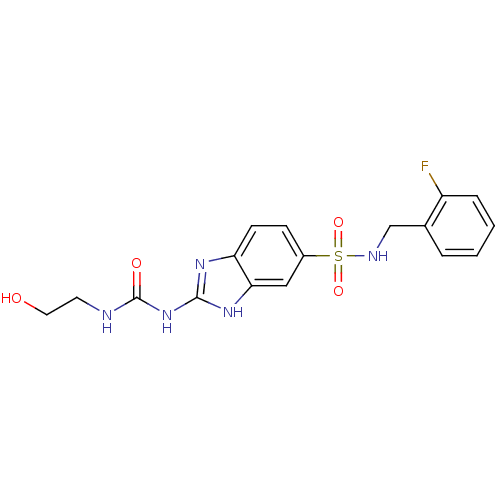
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chen, Q; Tian, W; Han, G; Qi, J; Zheng, C; Zhou, Y; Ding, L; Zhao, J; Zhu, J; Lv, J; Sheng, C Design and synthesis of novel benzoheterocyclic derivatives as human acrosin inhibitors by scaffold hopping. Eur J Med Chem59:176-82 (2013) [PubMed] Article
Chen, Q; Tian, W; Han, G; Qi, J; Zheng, C; Zhou, Y; Ding, L; Zhao, J; Zhu, J; Lv, J; Sheng, C Design and synthesis of novel benzoheterocyclic derivatives as human acrosin inhibitors by scaffold hopping. Eur J Med Chem59:176-82 (2013) [PubMed] Article