| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Aldo-keto reductase family 1 member C1 |
|---|
| Ligand | BDBM50396700 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_942522 (CHEMBL2346177) |
|---|
| IC50 | >30000±n/a nM |
|---|
| Citation |  Heinrich, DM; Flanagan, JU; Jamieson, SM; Silva, S; Rigoreau, LJ; Trivier, E; Raynham, T; Turnbull, AP; Denny, WA Synthesis and structure-activity relationships for 1-(4-(piperidin-1-ylsulfonyl)phenyl)pyrrolidin-2-ones as novel non-carboxylate inhibitors of the aldo-keto reductase enzyme AKR1C3. Eur J Med Chem62:738-44 (2013) [PubMed] Article Heinrich, DM; Flanagan, JU; Jamieson, SM; Silva, S; Rigoreau, LJ; Trivier, E; Raynham, T; Turnbull, AP; Denny, WA Synthesis and structure-activity relationships for 1-(4-(piperidin-1-ylsulfonyl)phenyl)pyrrolidin-2-ones as novel non-carboxylate inhibitors of the aldo-keto reductase enzyme AKR1C3. Eur J Med Chem62:738-44 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Aldo-keto reductase family 1 member C1 |
|---|
| Name: | Aldo-keto reductase family 1 member C1 |
|---|
| Synonyms: | 20-alpha-HSD | 20-alpha-Hydroxysteroid Dehydrogenase (AKR1C1) | AK1C1_HUMAN | AKR1C1 | Aldo-keto reductase family 1 member C1 (AK1C1) | Aldo-keto reductase family 1 member C1 (AK1C1a) | Aldo-keto reductase family 1 member C1 (AKR1C1) | Chlordecone reductase homolog HAKRC | DDH | DDH1 | High-affinity hepatic bile acid-binding protein | Trans-1,2-dihydrobenzene-1,2-diol dehydrogenase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 36793.97 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Recombinant AKR1C1 enzyme was expressed in E. coli. |
|---|
| Residue: | 323 |
|---|
| Sequence: | MDSKYQCVKLNDGHFMPVLGFGTYAPAEVPKSKALEATKLAIEAGFRHIDSAHLYNNEEQ
VGLAIRSKIADGSVKREDIFYTSKLWCNSHRPELVRPALERSLKNLQLDYVDLYLIHFPV
SVKPGEEVIPKDENGKILFDTVDLCATWEAVEKCKDAGLAKSIGVSNFNRRQLEMILNKP
GLKYKPVCNQVECHPYFNQRKLLDFCKSKDIVLVAYSALGSHREEPWVDPNSPVLLEDPV
LCALAKKHKRTPALIALRYQLQRGVVVLAKSYNEQRIRQNVQVFEFQLTSEEMKAIDGLN
RNVRYLTLDIFAGPPNYPFSDEY
|
|
|
|---|
| BDBM50396700 |
|---|
| n/a |
|---|
| Name | BDBM50396700 |
|---|
| Synonyms: | CHEMBL2172076 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H20N2O3S |
|---|
| Mol. Mass. | 356.439 |
|---|
| SMILES | O=C1CCCN1c1ccc(cc1)S(=O)(=O)N1CCc2ccccc2C1 |
|---|
| Structure | 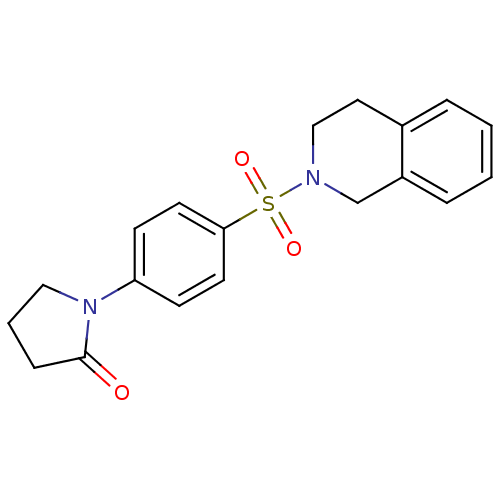
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Heinrich, DM; Flanagan, JU; Jamieson, SM; Silva, S; Rigoreau, LJ; Trivier, E; Raynham, T; Turnbull, AP; Denny, WA Synthesis and structure-activity relationships for 1-(4-(piperidin-1-ylsulfonyl)phenyl)pyrrolidin-2-ones as novel non-carboxylate inhibitors of the aldo-keto reductase enzyme AKR1C3. Eur J Med Chem62:738-44 (2013) [PubMed] Article
Heinrich, DM; Flanagan, JU; Jamieson, SM; Silva, S; Rigoreau, LJ; Trivier, E; Raynham, T; Turnbull, AP; Denny, WA Synthesis and structure-activity relationships for 1-(4-(piperidin-1-ylsulfonyl)phenyl)pyrrolidin-2-ones as novel non-carboxylate inhibitors of the aldo-keto reductase enzyme AKR1C3. Eur J Med Chem62:738-44 (2013) [PubMed] Article