| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM50436803 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_970665 (CHEMBL2405882) |
|---|
| IC50 | 9480±n/a nM |
|---|
| Citation |  Sivakumar, S; Ranjith Kumar, R; Ali, MA; Choon, TS An atom economic synthesis and AChE inhibitory activity of novel dispiro 7-aryltetrahydro-1H-pyrrolo[1,2-c][1,3]thiazole and 4-aryloctahydroindolizine N-methylpiperidin-4-one hybrid heterocycles. Eur J Med Chem65:240-8 (2013) [PubMed] Article Sivakumar, S; Ranjith Kumar, R; Ali, MA; Choon, TS An atom economic synthesis and AChE inhibitory activity of novel dispiro 7-aryltetrahydro-1H-pyrrolo[1,2-c][1,3]thiazole and 4-aryloctahydroindolizine N-methylpiperidin-4-one hybrid heterocycles. Eur J Med Chem65:240-8 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_ELEEL | Acetylcholinesterase (AChE) | Acetylcholinesterase (EeAChE) | ache |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 71812.79 |
|---|
| Organism: | Electrophorus electricus (Electric eel) |
|---|
| Description: | n/a |
|---|
| Residue: | 633 |
|---|
| Sequence: | MKILDALLFPVIFIMFFIHLSIAQTDPELTIMTRLGQVQGTRLPVPDRSHVIAFLGIPFA
EPPLGKMRFKPPEPKKPWNDVFDARDYPSACYQYVDTSYPGFSGTEMWNPNRMMSEDCLY
LNVWVPATPRPHNLTVMVWIYGGGFYSGSSSLDVYDGRYLAHSEKVVVVSMNYRVSAFGF
LALNGSAEAPGNVGLLDQRLALQWVQDNIHFFGGNPKQVTIFGESAGAASVGMHLLSPDS
RPKFTRAILQSGVPNGPWRTVSFDEARRRAIKLGRLVGCPDGNDTDLIDCLRSKQPQDLI
DQEWLVLPFSGLFRFSFVPVIDGVVFPDTPEAMLNSGNFKDTQILLGVNQNEGSYFLIYG
APGFSKDNESLITREDFLQGVKMSVPHANEIGLEAVILQYTDWMDEDNPIKNREAMDDIV
GDHNVVCPLQHFAKMYAQYSILQGQTGTASQGNLGWGNSGSASNSGNSQVSVYLYMFDHR
ASNLVWPEWMGVIHGYEIEFVFGLPLEKRLNYTLEEEKLSRRMMKYWANFARTGNPNINV
DGSIDSRRRWPVFTSTEQKHVGLNTDSLKVHKGLKSQFCALWNRFLPRLLNVTENIDDAE
RQWKAEFHRWSSYMMHWKNQFDHYSKQERCTNL
|
|
|
|---|
| BDBM50436803 |
|---|
| n/a |
|---|
| Name | BDBM50436803 |
|---|
| Synonyms: | CHEMBL3038152 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C43H34N2O2S |
|---|
| Mol. Mass. | 642.807 |
|---|
| SMILES | CN1C\C(=C/c2cccc3ccccc23)C(=O)[C@]2(C1)[C@H]([C@@H]1CSCN1[C@@]21C(=O)c2cccc3cccc1c23)c1cccc2ccccc12 |r| |
|---|
| Structure | 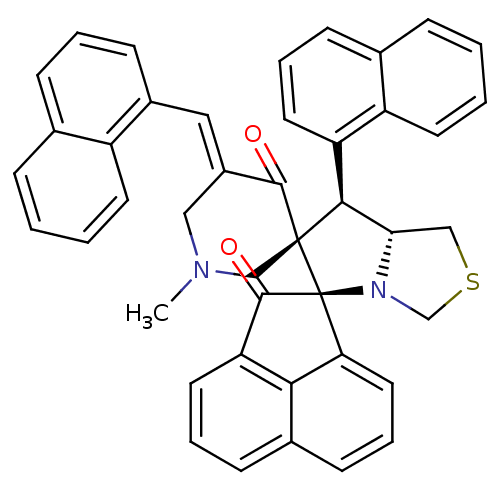
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sivakumar, S; Ranjith Kumar, R; Ali, MA; Choon, TS An atom economic synthesis and AChE inhibitory activity of novel dispiro 7-aryltetrahydro-1H-pyrrolo[1,2-c][1,3]thiazole and 4-aryloctahydroindolizine N-methylpiperidin-4-one hybrid heterocycles. Eur J Med Chem65:240-8 (2013) [PubMed] Article
Sivakumar, S; Ranjith Kumar, R; Ali, MA; Choon, TS An atom economic synthesis and AChE inhibitory activity of novel dispiro 7-aryltetrahydro-1H-pyrrolo[1,2-c][1,3]thiazole and 4-aryloctahydroindolizine N-methylpiperidin-4-one hybrid heterocycles. Eur J Med Chem65:240-8 (2013) [PubMed] Article