| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histone acetyltransferase KAT2B |
|---|
| Ligand | BDBM50445038 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1282380 (CHEMBL3100559) |
|---|
| IC50 | 2800±n/a nM |
|---|
| Citation |  van Ameijde, J; Zwiebel, AP; Ruijtenbeek, R; Liskamp, RM Azide-alkyne cycloaddition affording enzymatically tunable bisubstrate based inhibitors of histone acetyltransferase PCAF. Bioorg Med Chem Lett24:113-6 (2013) [PubMed] Article van Ameijde, J; Zwiebel, AP; Ruijtenbeek, R; Liskamp, RM Azide-alkyne cycloaddition affording enzymatically tunable bisubstrate based inhibitors of histone acetyltransferase PCAF. Bioorg Med Chem Lett24:113-6 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histone acetyltransferase KAT2B |
|---|
| Name: | Histone acetyltransferase KAT2B |
|---|
| Synonyms: | Histone acetyltransferase KAT2A/KAT2B | Histone acetyltransferase PCAF | KAT2B | KAT2B_HUMAN | PCAF |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 93045.76 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1502838 |
|---|
| Residue: | 832 |
|---|
| Sequence: | MSEAGGAGPGGCGAGAGAGAGPGALPPQPAALPPAPPQGSPCAAAAGGSGACGPATAVAA
AGTAEGPGGGGSARIAVKKAQLRSAPRAKKLEKLGVYSACKAEESCKCNGWKNPNPSPTP
PRADLQQIIVSLTESCRSCSHALAAHVSHLENVSEEEMNRLLGIVLDVEYLFTCVHKEED
ADTKQVYFYLFKLLRKSILQRGKPVVEGSLEKKPPFEKPSIEQGVNNFVQYKFSHLPAKE
RQTIVELAKMFLNRINYWHLEAPSQRRLRSPNDDISGYKENYTRWLCYCNVPQFCDSLPR
YETTQVFGRTLLRSVFTVMRRQLLEQARQEKDKLPLEKRTLILTHFPKFLSMLEEEVYSQ
NSPIWDQDFLSASSRTSQLGIQTVINPPPVAGTISYNSTSSSLEQPNAGSSSPACKASSG
LEANPGEKRKMTDSHVLEEAKKPRVMGDIPMELINEVMSTITDPAAMLGPETNFLSAHSA
RDEAARLEERRGVIEFHVVGNSLNQKPNKKILMWLVGLQNVFSHQLPRMPKEYITRLVFD
PKHKTLALIKDGRVIGGICFRMFPSQGFTEIVFCAVTSNEQVKGYGTHLMNHLKEYHIKH
DILNFLTYADEYAIGYFKKQGFSKEIKIPKTKYVGYIKDYEGATLMGCELNPRIPYTEFS
VIIKKQKEIIKKLIERKQAQIRKVYPGLSCFKDGVRQIPIESIPGIRETGWKPSGKEKSK
EPRDPDQLYSTLKSILQQVKSHQSAWPFMEPVKRTEAPGYYEVIRFPMDLKTMSERLKNR
YYVSKKLFMADLQRVFTNCKEYNPPESEYYKCANILEKFFFSKIKEAGLIDK
|
|
|
|---|
| BDBM50445038 |
|---|
| n/a |
|---|
| Name | BDBM50445038 |
|---|
| Synonyms: | CHEMBL3098705 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C78H136N31O31P3S |
|---|
| Mol. Mass. | 2129.09 |
|---|
| SMILES | [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-n1cc(-[#6]-[#16]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#7]-[#6](=O)-[#6@@H](-[#8])C([#6])([#6])[#6]-[#8]P([#8])(=O)[#8]P([#8])(=O)[#8]-[#6]-[#6@H]-2-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-2-[#8]P([#8])([#8])=O)-n2cnc3c(-[#7])ncnc23)nn1)-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| |
|---|
| Structure | 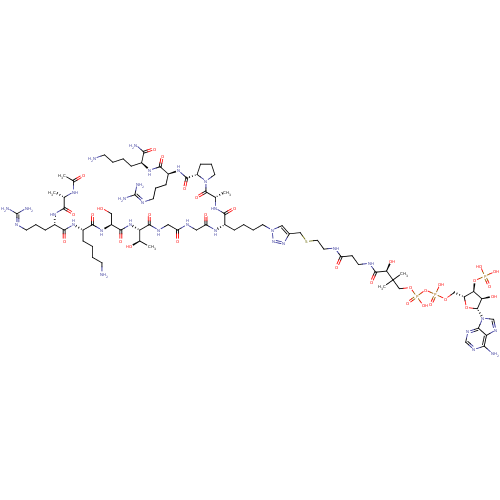
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 van Ameijde, J; Zwiebel, AP; Ruijtenbeek, R; Liskamp, RM Azide-alkyne cycloaddition affording enzymatically tunable bisubstrate based inhibitors of histone acetyltransferase PCAF. Bioorg Med Chem Lett24:113-6 (2013) [PubMed] Article
van Ameijde, J; Zwiebel, AP; Ruijtenbeek, R; Liskamp, RM Azide-alkyne cycloaddition affording enzymatically tunable bisubstrate based inhibitors of histone acetyltransferase PCAF. Bioorg Med Chem Lett24:113-6 (2013) [PubMed] Article