

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | P2X purinoceptor 4 | ||
| Ligand | BDBM50446063 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_1283892 (CHEMBL3106505) | ||
| IC50 | >10000±n/a nM | ||
| Citation |  Tian, M; Abdelrahman, A; Weinhausen, S; Hinz, S; Weyer, S; Dosa, S; El-Tayeb, A; Müller, CE Carbamazepine derivatives with P2X4 receptor-blocking activity. Bioorg Med Chem22:1077-88 (2014) [PubMed] Article Tian, M; Abdelrahman, A; Weinhausen, S; Hinz, S; Weyer, S; Dosa, S; El-Tayeb, A; Müller, CE Carbamazepine derivatives with P2X4 receptor-blocking activity. Bioorg Med Chem22:1077-88 (2014) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| P2X purinoceptor 4 | |||
| Name: | P2X purinoceptor 4 | ||
| Synonyms: | ATP receptor | P2RX4 | P2RX4_HUMAN | P2X4 | Purinergic receptor | Purinergic, P2X4 | ||
| Type: | Enzyme Catalytic Domain | ||
| Mol. Mass.: | 43374.70 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | Purinergic, P2X4 0 HUMAN::Q99571 | ||
| Residue: | 388 | ||
| Sequence: |
| ||
| BDBM50446063 | |||
| n/a | |||
| Name | BDBM50446063 | ||
| Synonyms: | CHEMBL3103382 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C21H23N3O | ||
| Mol. Mass. | 333.4268 | ||
| SMILES | NC1CCC(CC1)NC(=O)N1c2ccccc2C=Cc2ccccc12 |c:19,(34.96,-14.27,;34.97,-12.73,;33.64,-11.95,;33.64,-10.41,;34.99,-9.65,;36.31,-10.41,;36.3,-11.96,;34.99,-8.11,;33.65,-7.33,;32.33,-8.1,;33.66,-5.83,;32.3,-5.16,;31.17,-6.21,;29.7,-5.76,;29.35,-4.24,;30.49,-3.19,;31.96,-3.66,;32.92,-2.47,;34.46,-2.48,;35.4,-3.68,;36.86,-3.24,;37.98,-4.29,;37.63,-5.79,;36.15,-6.22,;35.04,-5.16,)| | ||
| Structure | 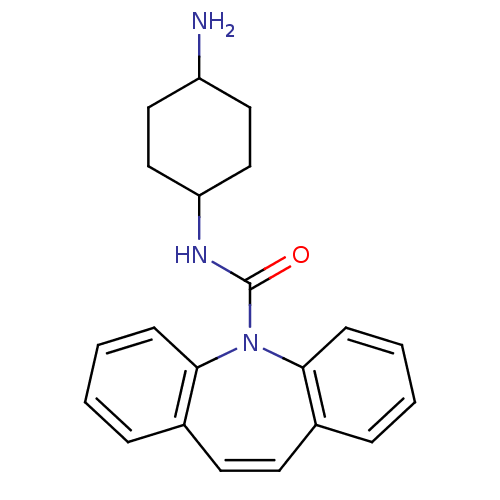
| ||