| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glutamate carboxypeptidase 2 |
|---|
| Ligand | BDBM50088186 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1476626 (CHEMBL3428582) |
|---|
| IC50 | 60000±n/a nM |
|---|
| Citation |  Ley, CR; Beattie, NR; Dannoon, S; Regan, M; VanBrocklin, H; Berkman, CE Synthesis and evaluation of constrained phosphoramidate inhibitors of prostate-specific membrane antigen. Bioorg Med Chem Lett25:2536-9 (2015) [PubMed] Article Ley, CR; Beattie, NR; Dannoon, S; Regan, M; VanBrocklin, H; Berkman, CE Synthesis and evaluation of constrained phosphoramidate inhibitors of prostate-specific membrane antigen. Bioorg Med Chem Lett25:2536-9 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glutamate carboxypeptidase 2 |
|---|
| Name: | Glutamate carboxypeptidase 2 |
|---|
| Synonyms: | FGCP | FOLH | FOLH1 | FOLH1_HUMAN | Folate hydrolase 1 | Folylpoly-gamma-glutamate carboxypeptidase | Glutamate carboxypeptidase 2 | Glutamate carboxypeptidase II | Membrane glutamate carboxypeptidase | N-acetylated-alpha-linked acidic dipeptidase I | NAALAD1 | NAALADase I | PSM | PSMA | Prostate-specific membrane antigen | Pteroylpoly-gamma-glutamate carboxypeptidase | mGCP |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 84333.66 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1497035 |
|---|
| Residue: | 750 |
|---|
| Sequence: | MWNLLHETDSAVATARRPRWLCAGALVLAGGFFLLGFLFGWFIKSSNEATNITPKHNMKA
FLDELKAENIKKFLYNFTQIPHLAGTEQNFQLAKQIQSQWKEFGLDSVELAHYDVLLSYP
NKTHPNYISIINEDGNEIFNTSLFEPPPPGYENVSDIVPPFSAFSPQGMPEGDLVYVNYA
RTEDFFKLERDMKINCSGKIVIARYGKVFRGNKVKNAQLAGAKGVILYSDPADYFAPGVK
SYPDGWNLPGGGVQRGNILNLNGAGDPLTPGYPANEYAYRRGIAEAVGLPSIPVHPIGYY
DAQKLLEKMGGSAPPDSSWRGSLKVPYNVGPGFTGNFSTQKVKMHIHSTNEVTRIYNVIG
TLRGAVEPDRYVILGGHRDSWVFGGIDPQSGAAVVHEIVRSFGTLKKEGWRPRRTILFAS
WDAEEFGLLGSTEWAEENSRLLQERGVAYINADSSIEGNYTLRVDCTPLMYSLVHNLTKE
LKSPDEGFEGKSLYESWTKKSPSPEFSGMPRISKLGSGNDFEVFFQRLGIASGRARYTKN
WETNKFSGYPLYHSVYETYELVEKFYDPMFKYHLTVAQVRGGMVFELANSIVLPFDCRDY
AVVLRKYADKIYSISMKHPQEMKTYSVSFDSLFSAVKNFTEIASKFSERLQDFDKSNPIV
LRMMNDQLMFLERAFIDPLGLPDRPFYRHVIYAPSSHNKYAGESFPGIYDALFDIESKVD
PSKAWGEVKRQIYVAAFTVQAAAETLSEVA
|
|
|
|---|
| BDBM50088186 |
|---|
| n/a |
|---|
| Name | BDBM50088186 |
|---|
| Synonyms: | CHEMBL3427436 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H24N3O12P |
|---|
| Mol. Mass. | 469.3377 |
|---|
| SMILES | N[C@@H](CCC(=O)N1C[C@@H](C[C@H]1C(O)=O)OP(O)(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O |r| |
|---|
| Structure | 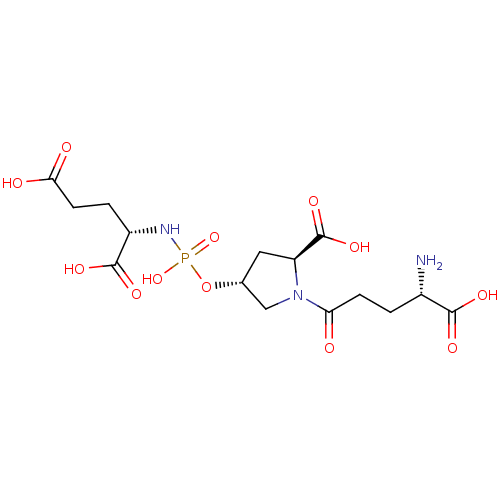
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ley, CR; Beattie, NR; Dannoon, S; Regan, M; VanBrocklin, H; Berkman, CE Synthesis and evaluation of constrained phosphoramidate inhibitors of prostate-specific membrane antigen. Bioorg Med Chem Lett25:2536-9 (2015) [PubMed] Article
Ley, CR; Beattie, NR; Dannoon, S; Regan, M; VanBrocklin, H; Berkman, CE Synthesis and evaluation of constrained phosphoramidate inhibitors of prostate-specific membrane antigen. Bioorg Med Chem Lett25:2536-9 (2015) [PubMed] Article