| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50132507 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1527451 (CHEMBL3636982) |
|---|
| IC50 | >30000±n/a nM |
|---|
| Citation |  Malosh, C; Turlington, M; Bridges, TM; Rook, JM; Noetzel, MJ; Vinson, PN; Steckler, T; Lavreysen, H; Mackie, C; Bartolomé-Nebreda, JM; Conde-Ceide, S; Martínez-Viturro, CM; Piedrafita, M; Sánchez-Casado, MR; Macdonald, GJ; Daniels, JS; Jones, CK; Niswender, CM; Conn, PJ; Lindsley, CW; Stauffer, SR Acyl dihydropyrazolo[1,5-a]pyrimidinones as metabotropic glutamate receptor 5 positive allosteric modulators. Bioorg Med Chem Lett25:5115-20 (2015) [PubMed] Article Malosh, C; Turlington, M; Bridges, TM; Rook, JM; Noetzel, MJ; Vinson, PN; Steckler, T; Lavreysen, H; Mackie, C; Bartolomé-Nebreda, JM; Conde-Ceide, S; Martínez-Viturro, CM; Piedrafita, M; Sánchez-Casado, MR; Macdonald, GJ; Daniels, JS; Jones, CK; Niswender, CM; Conn, PJ; Lindsley, CW; Stauffer, SR Acyl dihydropyrazolo[1,5-a]pyrimidinones as metabotropic glutamate receptor 5 positive allosteric modulators. Bioorg Med Chem Lett25:5115-20 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50132507 |
|---|
| n/a |
|---|
| Name | BDBM50132507 |
|---|
| Synonyms: | CHEMBL3633943 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H17N3O2 |
|---|
| Mol. Mass. | 271.3144 |
|---|
| SMILES | CC(=O)N1CCCn2nc(COc3ccccc3)cc12 |
|---|
| Structure | 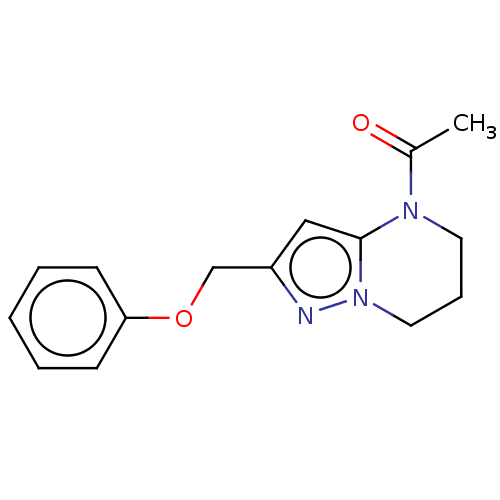
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Malosh, C; Turlington, M; Bridges, TM; Rook, JM; Noetzel, MJ; Vinson, PN; Steckler, T; Lavreysen, H; Mackie, C; Bartolomé-Nebreda, JM; Conde-Ceide, S; Martínez-Viturro, CM; Piedrafita, M; Sánchez-Casado, MR; Macdonald, GJ; Daniels, JS; Jones, CK; Niswender, CM; Conn, PJ; Lindsley, CW; Stauffer, SR Acyl dihydropyrazolo[1,5-a]pyrimidinones as metabotropic glutamate receptor 5 positive allosteric modulators. Bioorg Med Chem Lett25:5115-20 (2015) [PubMed] Article
Malosh, C; Turlington, M; Bridges, TM; Rook, JM; Noetzel, MJ; Vinson, PN; Steckler, T; Lavreysen, H; Mackie, C; Bartolomé-Nebreda, JM; Conde-Ceide, S; Martínez-Viturro, CM; Piedrafita, M; Sánchez-Casado, MR; Macdonald, GJ; Daniels, JS; Jones, CK; Niswender, CM; Conn, PJ; Lindsley, CW; Stauffer, SR Acyl dihydropyrazolo[1,5-a]pyrimidinones as metabotropic glutamate receptor 5 positive allosteric modulators. Bioorg Med Chem Lett25:5115-20 (2015) [PubMed] Article