| Reaction Details |
|---|
 | Report a problem with these data |
| Target | S-adenosylhomocysteine hydrolase-like protein 1 |
|---|
| Ligand | BDBM50140074 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1547422 (CHEMBL3757541) |
|---|
| IC50 | 2.7±n/a nM |
|---|
| Citation |  Liu, C; Chen, Q; Schneller, SW Enantiomeric 3-deaza-1',6'-isoneplanocin and its 3-bromo analogue: Synthesis by the Ullmann reaction and their antiviral properties. Bioorg Med Chem Lett26:928-30 (2016) [PubMed] Article Liu, C; Chen, Q; Schneller, SW Enantiomeric 3-deaza-1',6'-isoneplanocin and its 3-bromo analogue: Synthesis by the Ullmann reaction and their antiviral properties. Bioorg Med Chem Lett26:928-30 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| S-adenosylhomocysteine hydrolase-like protein 1 |
|---|
| Name: | S-adenosylhomocysteine hydrolase-like protein 1 |
|---|
| Synonyms: | AHCYL1 | Adenosylhomocysteinase 2 | AdoHcyase 2 | DC-expressed AHCY-like molecule | DCAL | DCAL | IP(3)Rs binding protein released with IP(3) | IRBIT | SAHH2_HUMAN | XPVKONA |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 58952.83 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_116703 |
|---|
| Residue: | 530 |
|---|
| Sequence: | MSMPDAMPLPGVGEELKQAKEIEDAEKYSFMATVTKAPKKQIQFADDMQEFTKFPTKTGR
RSLSRSISQSSTDSYSSAASYTDSSDDEVSPREKQQTNSKGSSNFCVKNIKQAEFGRREI
EIAEQDMSALISLRKRAQGEKPLAGAKIVGCTHITAQTAVLIETLCALGAQCRWSACNIY
STQNEVAAALAEAGVAVFAWKGESEDDFWWCIDRCVNMDGWQANMILDDGGDLTHWVYKK
YPNVFKKIRGIVEESVTGVHRLYQLSKAGKLCVPAMNVNDSVTKQKFDNLYCCRESILDG
LKRTTDVMFGGKQVVVCGYGEVGKGCCAALKALGAIVYITEIDPICALQACMDGFRVVKL
NEVIRQVDVVITCTGNKNVVTREHLDRMKNSCIVCNMGHSNTEIDVTSLRTPELTWERVR
SQVDHVIWPDGKRVVLLAEGRLLNLSCSTVPTFVLSITATTQALALIELYNAPEGRYKQD
VYLLPKKMDEYVASLHLPSFDAHLTELTDDQAKYLGLNKNGPFKPNYYRY
|
|
|
|---|
| BDBM50140074 |
|---|
| n/a |
|---|
| Name | BDBM50140074 |
|---|
| Synonyms: | CHEMBL2059155 | US10227373, Compound 3-Bromo-3-deazaneplanocin |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C12H13BrN4O3 |
|---|
| Mol. Mass. | 341.161 |
|---|
| SMILES | Nc1ncc(Br)c2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:14| |
|---|
| Structure | 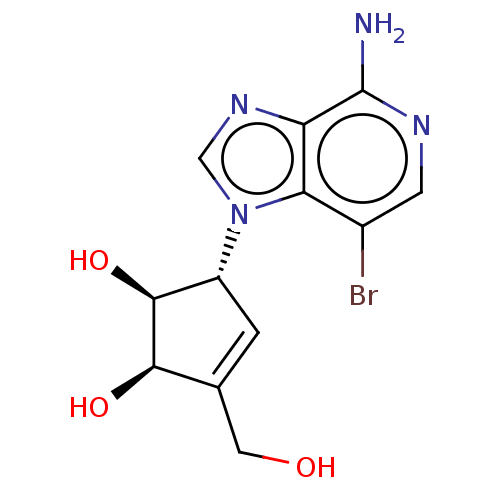
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Liu, C; Chen, Q; Schneller, SW Enantiomeric 3-deaza-1',6'-isoneplanocin and its 3-bromo analogue: Synthesis by the Ullmann reaction and their antiviral properties. Bioorg Med Chem Lett26:928-30 (2016) [PubMed] Article
Liu, C; Chen, Q; Schneller, SW Enantiomeric 3-deaza-1',6'-isoneplanocin and its 3-bromo analogue: Synthesis by the Ullmann reaction and their antiviral properties. Bioorg Med Chem Lett26:928-30 (2016) [PubMed] Article