| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Serine/threonine-protein kinase mTOR |
|---|
| Ligand | BDBM25116 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1587938 (CHEMBL3826741) |
|---|
| IC50 | 9318±n/a nM |
|---|
| Citation |  Fraser, C; Dawson, JC; Dowling, R; Houston, DR; Weiss, JT; Munro, AF; Muir, M; Harrington, L; Webster, SP; Frame, MC; Brunton, VG; Patton, EE; Carragher, NO; Unciti-Broceta, A Rapid Discovery and Structure-Activity Relationships of Pyrazolopyrimidines That Potently Suppress Breast Cancer Cell Growth via SRC Kinase Inhibition with Exceptional Selectivity over ABL Kinase. J Med Chem59:4697-710 (2016) [PubMed] Article Fraser, C; Dawson, JC; Dowling, R; Houston, DR; Weiss, JT; Munro, AF; Muir, M; Harrington, L; Webster, SP; Frame, MC; Brunton, VG; Patton, EE; Carragher, NO; Unciti-Broceta, A Rapid Discovery and Structure-Activity Relationships of Pyrazolopyrimidines That Potently Suppress Breast Cancer Cell Growth via SRC Kinase Inhibition with Exceptional Selectivity over ABL Kinase. J Med Chem59:4697-710 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Serine/threonine-protein kinase mTOR |
|---|
| Name: | Serine/threonine-protein kinase mTOR |
|---|
| Synonyms: | FK506-binding protein 12-rapamycin complex-associated protein 1 | FKBP12-rapamycin complex-associated protein | FRAP | FRAP 1 (mTOR) | FRAP1 | FRAP2 | MTOR | MTOR_HUMAN | Mammalian Target of Rapamycin (mTOR) | P42345 | RAFT1 | RAPT1 | Rapamycin and FKBP12 target 1 | Rapamycin target protein | Serine/threonine-protein kinase (mTOR) | mTORC2 |
|---|
| Type: | Rapamycin target protein |
|---|
| Mol. Mass.: | 288917.12 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P42345 |
|---|
| Residue: | 2549 |
|---|
| Sequence: | MLGTGPAAATTAATTSSNVSVLQQFASGLKSRNEETRAKAAKELQHYVTMELREMSQEES
TRFYDQLNHHIFELVSSSDANERKGGILAIASLIGVEGGNATRIGRFANYLRNLLPSNDP
VVMEMASKAIGRLAMAGDTFTAEYVEFEVKRALEWLGADRNEGRRHAAVLVLRELAISVP
TFFFQQVQPFFDNIFVAVWDPKQAIREGAVAALRACLILTTQREPKEMQKPQWYRHTFEE
AEKGFDETLAKEKGMNRDDRIHGALLILNELVRISSMEGERLREEMEEITQQQLVHDKYC
KDLMGFGTKPRHITPFTSFQAVQPQQSNALVGLLGYSSHQGLMGFGTSPSPAKSTLVESR
CCRDLMEEKFDQVCQWVLKCRNSKNSLIQMTILNLLPRLAAFRPSAFTDTQYLQDTMNHV
LSCVKKEKERTAAFQALGLLSVAVRSEFKVYLPRVLDIIRAALPPKDFAHKRQKAMQVDA
TVFTCISMLARAMGPGIQQDIKELLEPMLAVGLSPALTAVLYDLSRQIPQLKKDIQDGLL
KMLSLVLMHKPLRHPGMPKGLAHQLASPGLTTLPEASDVGSITLALRTLGSFEFEGHSLT
QFVRHCADHFLNSEHKEIRMEAARTCSRLLTPSIHLISGHAHVVSQTAVQVVADVLSKLL
VVGITDPDPDIRYCVLASLDERFDAHLAQAENLQALFVALNDQVFEIRELAICTVGRLSS
MNPAFVMPFLRKMLIQILTELEHSGIGRIKEQSARMLGHLVSNAPRLIRPYMEPILKALI
LKLKDPDPDPNPGVINNVLATIGELAQVSGLEMRKWVDELFIIIMDMLQDSSLLAKRQVA
LWTLGQLVASTGYVVEPYRKYPTLLEVLLNFLKTEQNQGTRREAIRVLGLLGALDPYKHK
VNIGMIDQSRDASAVSLSESKSSQDSSDYSTSEMLVNMGNLPLDEFYPAVSMVALMRIFR
DQSLSHHHTMVVQAITFIFKSLGLKCVQFLPQVMPTFLNVIRVCDGAIREFLFQQLGMLV
SFVKSHIRPYMDEIVTLMREFWVMNTSIQSTIILLIEQIVVALGGEFKLYLPQLIPHMLR
VFMHDNSPGRIVSIKLLAAIQLFGANLDDYLHLLLPPIVKLFDAPEAPLPSRKAALETVD
RLTESLDFTDYASRIIHPIVRTLDQSPELRSTAMDTLSSLVFQLGKKYQIFIPMVNKVLV
RHRINHQRYDVLICRIVKGYTLADEEEDPLIYQHRMLRSGQGDALASGPVETGPMKKLHV
STINLQKAWGAARRVSKDDWLEWLRRLSLELLKDSSSPSLRSCWALAQAYNPMARDLFNA
AFVSCWSELNEDQQDELIRSIELALTSQDIAEVTQTLLNLAEFMEHSDKGPLPLRDDNGI
VLLGERAAKCRAYAKALHYKELEFQKGPTPAILESLISINNKLQQPEAAAGVLEYAMKHF
GELEIQATWYEKLHEWEDALVAYDKKMDTNKDDPELMLGRMRCLEALGEWGQLHQQCCEK
WTLVNDETQAKMARMAAAAAWGLGQWDSMEEYTCMIPRDTHDGAFYRAVLALHQDLFSLA
QQCIDKARDLLDAELTAMAGESYSRAYGAMVSCHMLSELEEVIQYKLVPERREIIRQIWW
ERLQGCQRIVEDWQKILMVRSLVVSPHEDMRTWLKYASLCGKSGRLALAHKTLVLLLGVD
PSRQLDHPLPTVHPQVTYAYMKNMWKSARKIDAFQHMQHFVQTMQQQAQHAIATEDQQHK
QELHKLMARCFLKLGEWQLNLQGINESTIPKVLQYYSAATEHDRSWYKAWHAWAVMNFEA
VLHYKHQNQARDEKKKLRHASGANITNATTAATTAATATTTASTEGSNSESEAESTENSP
TPSPLQKKVTEDLSKTLLMYTVPAVQGFFRSISLSRGNNLQDTLRVLTLWFDYGHWPDVN
EALVEGVKAIQIDTWLQVIPQLIARIDTPRPLVGRLIHQLLTDIGRYHPQALIYPLTVAS
KSTTTARHNAANKILKNMCEHSNTLVQQAMMVSEELIRVAILWHEMWHEGLEEASRLYFG
ERNVKGMFEVLEPLHAMMERGPQTLKETSFNQAYGRDLMEAQEWCRKYMKSGNVKDLTQA
WDLYYHVFRRISKQLPQLTSLELQYVSPKLLMCRDLELAVPGTYDPNQPIIRIQSIAPSL
QVITSKQRPRKLTLMGSNGHEFVFLLKGHEDLRQDERVMQLFGLVNTLLANDPTSLRKNL
SIQRYAVIPLSTNSGLIGWVPHCDTLHALIRDYREKKKILLNIEHRIMLRMAPDYDHLTL
MQKVEVFEHAVNNTAGDDLAKLLWLKSPSSEVWFDRRTNYTRSLAVMSMVGYILGLGDRH
PSNLMLDRLSGKILHIDFGDCFEVAMTREKFPEKIPFRLTRMLTNAMEVTGLDGNYRITC
HTVMEVLREHKDSVMAVLEAFVYDPLLNWRLMDTNTKGNKRSRTRTDSYSAGQSVEILDG
VELGEPAHKKTGTTVPESIHSFIGDGLVKPEALNKKAIQIINRVRDKLTGRDFSHDDTLD
VPTQVELLIKQATSHENLCQCYIGWCPFW
|
|
|
|---|
| BDBM25116 |
|---|
| n/a |
|---|
| Name | BDBM25116 |
|---|
| Synonyms: | 1-tert-butyl-3-(4-methylphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine | CHEMBL306380 | PP1 | US10688093, Compound PP1 (Pfizer) | US10807986, Comparative compound 1 | US11046696, Compound 1 of Comparative Examples | US11701353, PP1 (Pfizer) | cid_1400 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H19N5 |
|---|
| Mol. Mass. | 281.3556 |
|---|
| SMILES | Cc1ccc(cc1)-c1nn(c2ncnc(N)c12)C(C)(C)C |
|---|
| Structure | 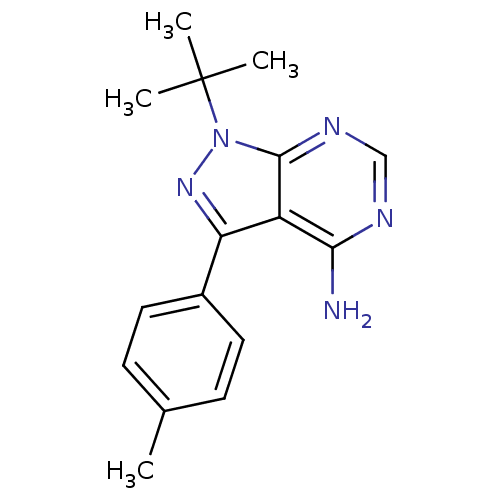
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Fraser, C; Dawson, JC; Dowling, R; Houston, DR; Weiss, JT; Munro, AF; Muir, M; Harrington, L; Webster, SP; Frame, MC; Brunton, VG; Patton, EE; Carragher, NO; Unciti-Broceta, A Rapid Discovery and Structure-Activity Relationships of Pyrazolopyrimidines That Potently Suppress Breast Cancer Cell Growth via SRC Kinase Inhibition with Exceptional Selectivity over ABL Kinase. J Med Chem59:4697-710 (2016) [PubMed] Article
Fraser, C; Dawson, JC; Dowling, R; Houston, DR; Weiss, JT; Munro, AF; Muir, M; Harrington, L; Webster, SP; Frame, MC; Brunton, VG; Patton, EE; Carragher, NO; Unciti-Broceta, A Rapid Discovery and Structure-Activity Relationships of Pyrazolopyrimidines That Potently Suppress Breast Cancer Cell Growth via SRC Kinase Inhibition with Exceptional Selectivity over ABL Kinase. J Med Chem59:4697-710 (2016) [PubMed] Article