

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Chymotrypsin-C | ||
| Ligand | BDBM87060 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Enzyme Inhibition Assay | ||
| pH | 7.6±0 | ||
| Temperature | 298.15±0 K | ||
| Ki | 4.711e+4± 7.2e+2 nM | ||
| Citation |  Arif Lodhi, M; Iqbal Choudhary, M; Malik, A; Ahmad, S alpha-Chymotrypsin inhibition studies on the lignans from Vitex negundo Linn. J Enzyme Inhib Med Chem23:400-5 (2008) [PubMed] Article Arif Lodhi, M; Iqbal Choudhary, M; Malik, A; Ahmad, S alpha-Chymotrypsin inhibition studies on the lignans from Vitex negundo Linn. J Enzyme Inhib Med Chem23:400-5 (2008) [PubMed] Article | ||
| More Info.: | Get all data from this article, Solution Info, Assay Method | ||
| Chymotrypsin-C | |||
| Name: | Chymotrypsin-C | ||
| Synonyms: | CLCR | CTRC | CTRC_HUMAN | Caldecrin | Chymotrypsin | Chymotrypsin C | Chymotrypsin-C | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 29487.98 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | Q99895 | ||
| Residue: | 268 | ||
| Sequence: |
| ||
| BDBM87060 | |||
| n/a | |||
| Name | BDBM87060 | ||
| Synonyms: | Lignan, 4 | MLS001138823 | N-(4-methylcyclohexyl)-3-(4-methylpiperazin-1-yl)sulfonyl-benzamide | N-(4-methylcyclohexyl)-3-(4-methylpiperazin-1-yl)sulfonylbenzamide | N-(4-methylcyclohexyl)-3-(4-methylpiperazino)sulfonyl-benzamide | N-(4-methylcyclohexyl)-3-[(4-methyl-1-piperazinyl)sulfonyl]benzamide | SMR000715032 | cid_24983123 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C19H29N3O3S | ||
| Mol. Mass. | 379.517 | ||
| SMILES | CC1CCC(CC1)NC(=O)c1cccc(c1)S(=O)(=O)N1CCN(C)CC1 |(3.08,-7.7,;4.41,-6.93,;5.75,-7.7,;7.08,-6.93,;7.08,-5.39,;5.75,-4.62,;4.41,-5.39,;8.41,-4.62,;8.41,-3.08,;7.08,-2.31,;9.75,-2.31,;11.08,-3.08,;12.42,-2.31,;12.42,-.77,;11.08,,;9.75,-.77,;11.08,1.54,;12.62,1.54,;9.54,1.54,;11.08,3.08,;12.42,3.85,;12.42,5.39,;11.08,6.16,;11.08,7.7,;9.75,5.39,;9.75,3.85,)| | ||
| Structure | 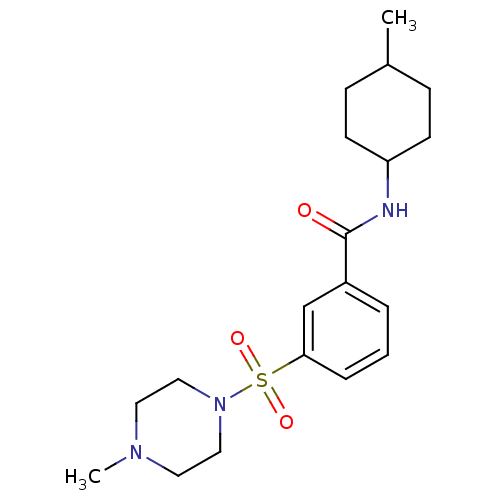
| ||