| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Serine protease 1 |
|---|
| Ligand | BDBM50110015 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Enzyme Assay |
|---|
| Temperature | 297.15±0 K |
|---|
| Ki | 150±0.0 nM |
|---|
| Citation |  Stuerzebecher, J; Steinmetzer, T; Schweinitz, A Urokinase inhibitors, production and use thereof US Patent US8476306 Publication Date 7/2/2013 Stuerzebecher, J; Steinmetzer, T; Schweinitz, A Urokinase inhibitors, production and use thereof US Patent US8476306 Publication Date 7/2/2013 |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Serine protease 1 |
|---|
| Name: | Serine protease 1 |
|---|
| Synonyms: | PTRYI | Prss1 | RATPTRYI | TRY1_RAT | Trp1 | Try1 | Tryp1 | Trypsin |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 25951.67 |
|---|
| Organism: | Rattus norvegicus |
|---|
| Description: | P00762 |
|---|
| Residue: | 246 |
|---|
| Sequence: | MSALLILALVGAAVAFPLEDDDKIVGGYTCPEHSVPYQVSLNSGYHFCGGSLINDQWVVS
AAHCYKSRIQVRLGEHNINVLEGDEQFINAAKIIKHPNYSSWTLNNDIMLIKLSSPVKLN
ARVAPVALPSACAPAGTQCLISGWGNTLSNGVNNPDLLQCVDAPVLSQADCEAAYPGEIT
SSMICVGFLEGGKDSCQGDSGGPVVCNGQLQGIVSWGYGCALPDNPGVYTKVCNFVGWIQ
DTIAAN
|
|
|
|---|
| BDBM50110015 |
|---|
| n/a |
|---|
| Name | BDBM50110015 |
|---|
| Synonyms: | CHEMBL158936 | N-(BENZYLSULFONYL)SERYL-N~1~-{4-[AMINO(IMINO)METHYL]BENZYL}GLYCINAMIDE | N-[(4-Carbamimidoyl-benzylcarbamoyl)-methyl]-3-hydroxy-2-phenylmethanesulfonylamino-propionamide | US8476306, 6.1 | US8476306, 6.2 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H25N5O5S |
|---|
| Mol. Mass. | 447.508 |
|---|
| SMILES | NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 |
|---|
| Structure | 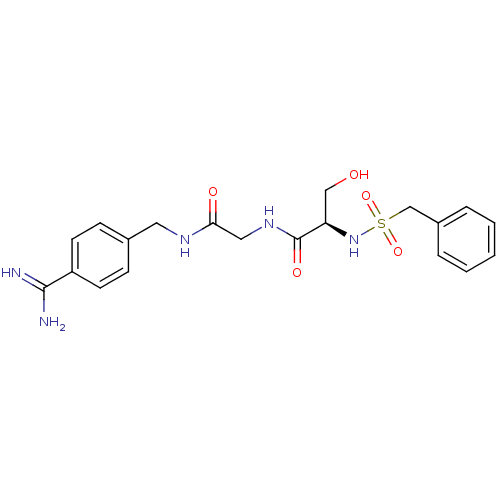
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Stuerzebecher, J; Steinmetzer, T; Schweinitz, A Urokinase inhibitors, production and use thereof US Patent US8476306 Publication Date 7/2/2013
Stuerzebecher, J; Steinmetzer, T; Schweinitz, A Urokinase inhibitors, production and use thereof US Patent US8476306 Publication Date 7/2/2013