| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Nitric oxide synthase, brain |
|---|
| Ligand | BDBM29234 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Enzyme Inhibition Assay |
|---|
| Ki | 85±n/a nM |
|---|
| Citation |  Silverman, RB; Meyskens, FL; Yang, S; Ji, H; Xue, F; Poulos, TL Specific nNOS inhibitors for the therapy and prevention of human melanoma US Patent US9090589 Publication Date 7/28/2015 Silverman, RB; Meyskens, FL; Yang, S; Ji, H; Xue, F; Poulos, TL Specific nNOS inhibitors for the therapy and prevention of human melanoma US Patent US9090589 Publication Date 7/28/2015 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Nitric oxide synthase, brain |
|---|
| Name: | Nitric oxide synthase, brain |
|---|
| Synonyms: | Constitutive NOS | N-NOS | NC-NOS | NOS type I | NOS type I nNOS | NOS1 | NOS1_HUMAN | Neuronal NOS | Neuronal nitric oxide synthase | Nitric oxide synthase, brain (nNOS) | Nitric oxide synthase, neuronal (nNOS) | Peptidyl-cysteine S-nitrosylase NOS1 | bNOS | nNOS |
|---|
| Type: | Homodimer |
|---|
| Mol. Mass.: | 160985.98 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P29475 |
|---|
| Residue: | 1434 |
|---|
| Sequence: | MEDHMFGVQQIQPNVISVRLFKRKVGGLGFLVKERVSKPPVIISDLIRGGAAEQSGLIQA
GDIILAVNGRPLVDLSYDSALEVLRGIASETHVVLILRGPEGFTTHLETTFTGDGTPKTI
RVTQPLGPPTKAVDLSHQPPAGKEQPLAVDGASGPGNGPQHAYDDGQEAGSLPHANGLAP
RPPGQDPAKKATRVSLQGRGENNELLKEIEPVLSLLTSGSRGVKGGAPAKAEMKDMGIQV
DRDLDGKSHKPLPLGVENDRVFNDLWGKGNVPVVLNNPYSEKEQPPTSGKQSPTKNGSPS
KCPRFLKVKNWETEVVLTDTLHLKSTLETGCTEYICMGSIMHPSQHARRPEDVRTKGQLF
PLAKEFIDQYYSSIKRFGSKAHMERLEEVNKEIDTTSTYQLKDTELIYGAKHAWRNASRC
VGRIQWSKLQVFDARDCTTAHGMFNYICNHVKYATNKGNLRSAITIFPQRTDGKHDFRVW
NSQLIRYAGYKQPDGSTLGDPANVQFTEICIQQGWKPPRGRFDVLPLLLQANGNDPELFQ
IPPELVLEVPIRHPKFEWFKDLGLKWYGLPAVSNMLLEIGGLEFSACPFSGWYMGTEIGV
RDYCDNSRYNILEEVAKKMNLDMRKTSSLWKDQALVEINIAVLYSFQSDKVTIVDHHSAT
ESFIKHMENEYRCRGGCPADWVWIVPPMSGSITPVFHQEMLNYRLTPSFEYQPDPWNTHV
WKGTNGTPTKRRAIGFKKLAEAVKFSAKLMGQAMAKRVKATILYATETGKSQAYAKTLCE
IFKHAFDAKVMSMEEYDIVHLEHETLVLVVTSTFGNGDPPENGEKFGCALMEMRHPNSVQ
EERKSYKVRFNSVSSYSDSQKSSGDGPDLRDNFESAGPLANVRFSVFGLGSRAYPHFCAF
GHAVDTLLEELGGERILKMREGDELCGQEEAFRTWAKKVFKAACDVFCVGDDVNIEKANN
SLISNDRSWKRNKFRLTFVAEAPELTQGLSNVHKKRVSAARLLSRQNLQSPKSSRSTIFV
RLHTNGSQELQYQPGDHLGVFPGNHEDLVNALIERLEDAPPVNQMVKVELLEERNTALGV
ISNWTDELRLPPCTIFQAFKYYLDITTPPTPLQLQQFASLATSEKEKQRLLVLSKGLQEY
EEWKWGKNPTIVEVLEEFPSIQMPATLLLTQLSLLQPRYYSISSSPDMYPDEVHLTVAIV
SYRTRDGEGPIHHGVCSSWLNRIQADELVPCFVRGAPSFHLPRNPQVPCILVGPGTGIAP
FRSFWQQRQFDIQHKGMNPCPMVLVFGCRQSKIDHIYREETLQAKNKGVFRELYTAYSRE
PDKPKKYVQDILQEQLAESVYRALKEQGGHIYVCGDVTMAADVLKAIQRIMTQQGKLSAE
DAGVFISRMRDDNRYHEDIFGVTLRTYEVTNRLRSESIAFIEESKKDTDEVFSS
|
|
|
|---|
| BDBM29234 |
|---|
| n/a |
|---|
| Name | BDBM29234 |
|---|
| Synonyms: | CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrolidine, 6 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H28ClN5 |
|---|
| Mol. Mass. | 373.923 |
|---|
| SMILES | Cc1cc(N)nc(C[C@H]2CNC[C@H]2NCCNCc2ccc(Cl)cc2)c1 |r| |
|---|
| Structure | 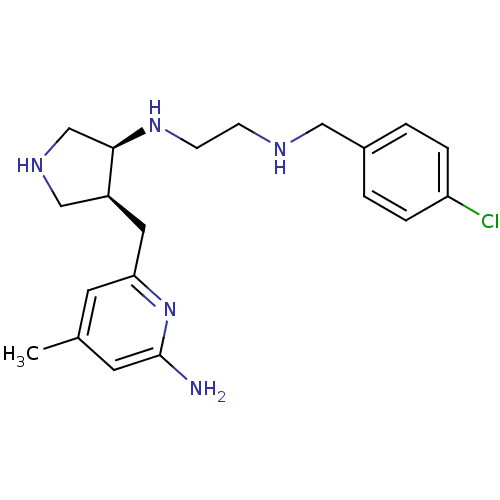
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Silverman, RB; Meyskens, FL; Yang, S; Ji, H; Xue, F; Poulos, TL Specific nNOS inhibitors for the therapy and prevention of human melanoma US Patent US9090589 Publication Date 7/28/2015
Silverman, RB; Meyskens, FL; Yang, S; Ji, H; Xue, F; Poulos, TL Specific nNOS inhibitors for the therapy and prevention of human melanoma US Patent US9090589 Publication Date 7/28/2015