| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM202363 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Inhibition Assay |
|---|
| pH | 8±n/a |
|---|
| Temperature | 298.15±n/a K |
|---|
| IC50 | 3.6±0.2 nM |
|---|
| Comments | extracted |
|---|
| Citation |  Muñoz-Torrero López-Ibarra, D; Inestrosa Cantín, NM; Viayna Gaza, E; Sola Lao, I; Vázquez Cruz, S Beta-amyloid-directed multitarget compounds for the treatment of alzheimer's disease US Patent US9238626 Publication Date 1/19/2016 Muñoz-Torrero López-Ibarra, D; Inestrosa Cantín, NM; Viayna Gaza, E; Sola Lao, I; Vázquez Cruz, S Beta-amyloid-directed multitarget compounds for the treatment of alzheimer's disease US Patent US9238626 Publication Date 1/19/2016 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_HUMAN | ACHE | Acetylcholinesterase (AChE) | Acetylcholinesterase (human AChE) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 67792.70 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P22303 |
|---|
| Residue: | 614 |
|---|
| Sequence: | MRPPQCLLHTPSLASPLLLLLLWLLGGGVGAEGREDAELLVTVRGGRLRGIRLKTPGGPV
SAFLGIPFAEPPMGPRRFLPPEPKQPWSGVVDATTFQSVCYQYVDTLYPGFEGTEMWNPN
RELSEDCLYLNVWTPYPRPTSPTPVLVWIYGGGFYSGASSLDVYDGRFLVQAERTVLVSM
NYRVGAFGFLALPGSREAPGNVGLLDQRLALQWVQENVAAFGGDPTSVTLFGESAGAASV
GMHLLSPPSRGLFHRAVLQSGAPNGPWATVGMGEARRRATQLAHLVGCPPGGTGGNDTEL
VACLRTRPAQVLVNHEWHVLPQESVFRFSFVPVVDGDFLSDTPEALINAGDFHGLQVLVG
VVKDEGSYFLVYGAPGFSKDNESLISRAEFLAGVRVGVPQVSDLAAEAVVLHYTDWLHPE
DPARLREALSDVVGDHNVVCPVAQLAGRLAAQGARVYAYVFEHRASTLSWPLWMGVPHGY
EIEFIFGIPLDPSRNYTAEEKIFAQRLMRYWANFARTGDPNEPRDPKAPQWPPYTAGAQQ
YVSLDLRPLEVRRGLRAQACAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKN
QFDHYSKQDRCSDL
|
|
|
|---|
| BDBM202363 |
|---|
| n/a |
|---|
| Name | BDBM202363 |
|---|
| Synonyms: | US9238626, (+/-)-(Ib) HCl |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C41H42ClN3O5 |
|---|
| Mol. Mass. | 692.242 |
|---|
| SMILES | CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCCCCNC(=O)c1cc(O)c2C(=O)c3c(O)cccc3C(=O)c2c1 |t:1| |
|---|
| Structure | 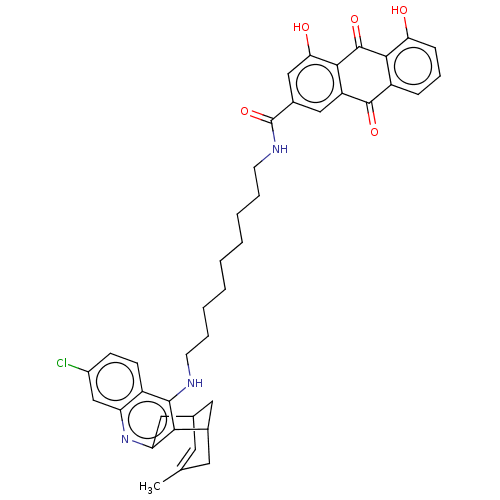
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Muñoz-Torrero López-Ibarra, D; Inestrosa Cantín, NM; Viayna Gaza, E; Sola Lao, I; Vázquez Cruz, S Beta-amyloid-directed multitarget compounds for the treatment of alzheimer's disease US Patent US9238626 Publication Date 1/19/2016
Muñoz-Torrero López-Ibarra, D; Inestrosa Cantín, NM; Viayna Gaza, E; Sola Lao, I; Vázquez Cruz, S Beta-amyloid-directed multitarget compounds for the treatment of alzheimer's disease US Patent US9238626 Publication Date 1/19/2016