| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Carbonic anhydrase 2 |
|---|
| Ligand | BDBM11626 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | CA Inhibition Assay |
|---|
| pH | 8.3±0 |
|---|
| Temperature | 298.15±0 K |
|---|
| Ki | 4.3e+2±n/a nM |
|---|
| Citation |  Maresca, A; Scozzafava, A; Vullo, D; Supuran, CT Dihalogenated sulfanilamides and benzolamides are effective inhibitors of the three ß-class carbonic anhydrases from Mycobacterium tuberculosis. J Enzyme Inhib Med Chem28:384-7 (2013) [PubMed] Article Maresca, A; Scozzafava, A; Vullo, D; Supuran, CT Dihalogenated sulfanilamides and benzolamides are effective inhibitors of the three ß-class carbonic anhydrases from Mycobacterium tuberculosis. J Enzyme Inhib Med Chem28:384-7 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Carbonic anhydrase 2 |
|---|
| Name: | Carbonic anhydrase 2 |
|---|
| Synonyms: | β-Carbonic anhydrase 2 (CA 2) | Carbonic anhydrase | MTCA2_MYCTU | canB | cynT | mtcA2 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 21788.97 |
|---|
| Organism: | Mycobacterium tuberculosis |
|---|
| Description: | P9WPJ9 |
|---|
| Residue: | 207 |
|---|
| Sequence: | MPNTNPVAAWKALKEGNERFVAGRPQHPSQSVDHRAGLAAGQKPTAVIFGCADSRVAAEI
IFDQGLGDMFVVRTAGHVIDSAVLGSIEYAVTVLNVPLIVVLGHDSCGAVNAALAAINDG
TLPGGYVRDVVERVAPSVLLGRRDGLSRVDEFEQRHVHETVAILMARSSAISERIAGGSL
AIVGVTYQLDDGRAVLRDHIGNIGEEV
|
|
|
|---|
| BDBM11626 |
|---|
| n/a |
|---|
| Name | BDBM11626 |
|---|
| Synonyms: | β-CA inhibitor, 2 | 2-N-(4-amino-3-bromo-5-fluorobenzene)-1,3,4-thiadiazole-2,5-disulfonamide | 5-{[(4-amino-3-bromo-5-fluorophenyl)sulfonyl]amino}-1,3,4-thiadiazole-2-sulfonamide | aminobenzolamide 19 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C8H7BrFN5O4S3 |
|---|
| Mol. Mass. | 432.27 |
|---|
| SMILES | Nc1c(F)cc(cc1Br)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O |
|---|
| Structure | 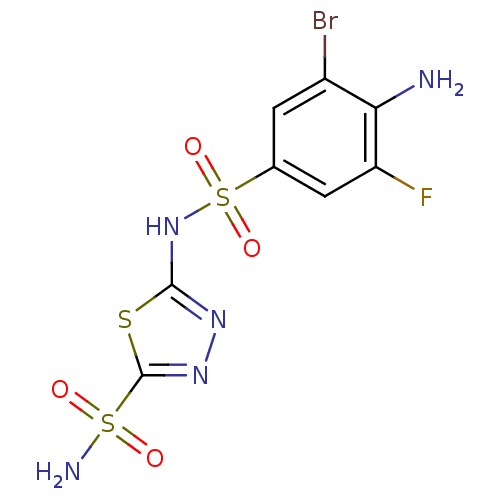
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Maresca, A; Scozzafava, A; Vullo, D; Supuran, CT Dihalogenated sulfanilamides and benzolamides are effective inhibitors of the three ß-class carbonic anhydrases from Mycobacterium tuberculosis. J Enzyme Inhib Med Chem28:384-7 (2013) [PubMed] Article
Maresca, A; Scozzafava, A; Vullo, D; Supuran, CT Dihalogenated sulfanilamides and benzolamides are effective inhibitors of the three ß-class carbonic anhydrases from Mycobacterium tuberculosis. J Enzyme Inhib Med Chem28:384-7 (2013) [PubMed] Article