| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor-type tyrosine-protein kinase FLT3 [D835H] |
|---|
| Ligand | BDBM247371 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | KINOMEScan Technology Assays |
|---|
| Kd | 11±n/a nM |
|---|
| Citation |  Blagg, J; Bavetsias, V; Moore, AS; Linardopoulos, S Pharmaceutically active compounds US Patent US9447092 Publication Date 9/20/2016 Blagg, J; Bavetsias, V; Moore, AS; Linardopoulos, S Pharmaceutically active compounds US Patent US9447092 Publication Date 9/20/2016 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor-type tyrosine-protein kinase FLT3 [D835H] |
|---|
| Name: | Receptor-type tyrosine-protein kinase FLT3 [D835H] |
|---|
| Synonyms: | CD135 | FL cytokine receptor | FLK2 | FLT3 | FLT3(D835H) | FLT3_HUMAN | Receptor-type tyrosine-protein kinase FLT3 (D835H) | STK-1 | STK1 | Stem cell tyrosine kinase 1 | Tyrosine Kinase FLT3 Mutant (D835H) |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 112912.18 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P36888 D835H |
|---|
| Residue: | 993 |
|---|
| Sequence: | MPALARDGGQLPLLVVFSAMIFGTITNQDLPVIKCVLINHKNNDSSVGKSSSYPMVSESP
EDLGCALRPQSSGTVYEAAAVEVDVSASITLQVLVDAPGNISCLWVFKHSSLNCQPHFDL
QNRGVVSMVILKMTETQAGEYLLFIQSEATNYTILFTVSIRNTLLYTLRRPYFRKMENQD
ALVCISESVPEPIVEWVLCDSQGESCKEESPAVVKKEEKVLHELFGTDIRCCARNELGRE
CTRLFTIDLNQTPQTTLPQLFLKVGEPLWIRCKAVHVNHGFGLTWELENKALEEGNYFEM
STYSTNRTMIRILFAFVSSVARNDTGYYTCSSSKHPSQSALVTIVEKGFINATNSSEDYE
IDQYEEFCFSVRFKAYPQIRCTWTFSRKSFPCEQKGLDNGYSISKFCNHKHQPGEYIFHA
ENDDAQFTKMFTLNIRRKPQVLAEASASQASCFSDGYPLPSWTWKKCSDKSPNCTEEITE
GVWNRKANRKVFGQWVSSSTLNMSEAIKGFLVKCCAYNSLGTSCETILLNSPGPFPFIQD
NISFYATIGVCLLFIVVLTLLICHKYKKQFRYESQLQMVQVTGSSDNEYFYVDFREYEYD
LKWEFPRENLEFGKVLGSGAFGKVMNATAYGISKTGVSIQVAVKMLKEKADSSEREALMS
ELKMMTQLGSHENIVNLLGACTLSGPIYLIFEYCCYGDLLNYLRSKREKFHRTWTEIFKE
HNFSFYPTFQSHPNSSMPGSREVQIHPDSDQISGLHGNSFHSEDEIEYENQKRLEEEEDL
NVLTFEDLLCFAYQVAKGMEFLEFKSCVHRDLAARNVLVTHGKVVKICDFGLARHIMSDS
NYVVRGNARLPVKWMAPESLFEGIYTIKSDVWSYGILLWEIFSLGVNPYPGIPVDANFYK
LIQNGFKMDQPFYATEEIYIIMQSCWAFDSRKRPSFPNLTSFLGCQLADAEEAMYQNVDG
RVSECPHTYQNRRPFSREMDLGLLSPQAQVEDS
|
|
|
|---|
| BDBM247371 |
|---|
| n/a |
|---|
| Name | BDBM247371 |
|---|
| Synonyms: | US9447092, 1 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H23Cl2N7 |
|---|
| Mol. Mass. | 456.371 |
|---|
| SMILES | Cc1nn(C)cc1-c1nc2c(N3CCN(Cc4ccc(Cl)cc4)CC3)c(Cl)cnc2[nH]1 |
|---|
| Structure | 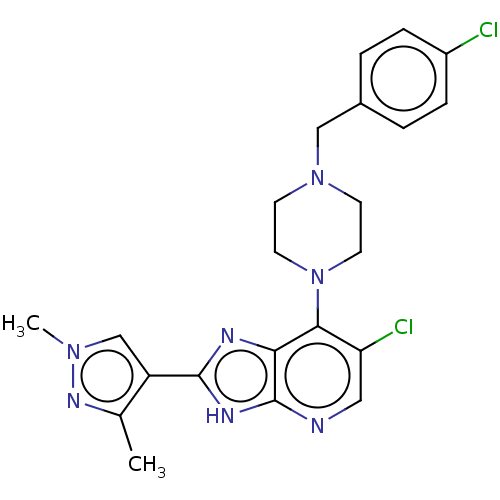
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Blagg, J; Bavetsias, V; Moore, AS; Linardopoulos, S Pharmaceutically active compounds US Patent US9447092 Publication Date 9/20/2016
Blagg, J; Bavetsias, V; Moore, AS; Linardopoulos, S Pharmaceutically active compounds US Patent US9447092 Publication Date 9/20/2016