| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Epoxide hydrolase |
|---|
| Ligand | BDBM25738 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Inhibition Assay |
|---|
| IC50 | 23.0±n/a nM |
|---|
| Citation |  Guedes, AG; Hammock, BD; Morisseau, C Treatment of inflammatory disorders in non-human mammals US Patent US10383835 Publication Date 8/20/2019 Guedes, AG; Hammock, BD; Morisseau, C Treatment of inflammatory disorders in non-human mammals US Patent US10383835 Publication Date 8/20/2019 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Epoxide hydrolase |
|---|
| Name: | Epoxide hydrolase |
|---|
| Synonyms: | EPHX1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 48577.06 |
|---|
| Organism: | Equus caballus (Horse) |
|---|
| Description: | A0A5F5PR84 |
|---|
| Residue: | 462 |
|---|
| Sequence: | MMTSLRVMWLEILLTSVLGFVIYWFVSRDKEETLPLEDGWWGPGSRPTGREDESIRPFKV
ETSDEEINDLHQRIDKARLTPPLEDSRFHYGFNSNYLKQIVSYWRNEFDWKKQVEILNRY
PHFKTKIEGLDIHFIHVKPPQLPSGRTPKPLLMVHGWPGSFYEFYKIIPFLTDPKSHGLG
DEHVFEVICPCIPGYGFSEASSKKGFNTVATARIFYKLMLRLGFQEFYVQGGDWGALICT
NIAQLVPSHVKGLHLNMAFVLRSFYTLTLPLGRHFAGLFGYTHKDVELMYPFKEKIFYSL
MRESGYMHIQATKPDTVGCALNDSPVGLAAYILEKFSTWTKSEFRDLEDGGLERKFSLDD
LLTNIMLYWTTGSITSSQRFYKENLGQGYMAHKHERMKVYVPTGFAAFPCELLHAPENWV
KFKYPKLISYSYMPRGGHFAAFEEPQLLAQDIRKFVGLLERQ
|
|
|
|---|
| BDBM25738 |
|---|
| n/a |
|---|
| Name | BDBM25738 |
|---|
| Synonyms: | 1-adamantan-1-yl-3-{5-[2-(2-ethoxyethoxy)ethoxy]pentyl}urea | CHEMBL242655 | US10383835, Compound 950 | Urea-based compound, 19 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H40N2O4 |
|---|
| Mol. Mass. | 396.564 |
|---|
| SMILES | CCOCCOCCOCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| |
|---|
| Structure | 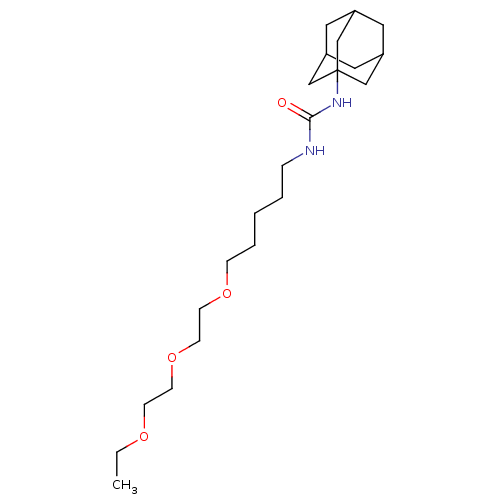
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Guedes, AG; Hammock, BD; Morisseau, C Treatment of inflammatory disorders in non-human mammals US Patent US10383835 Publication Date 8/20/2019
Guedes, AG; Hammock, BD; Morisseau, C Treatment of inflammatory disorders in non-human mammals US Patent US10383835 Publication Date 8/20/2019