| Reaction Details |
|---|
 | Report a problem with these data |
| Target | UL37 immediate early glycoprotein |
|---|
| Ligand | BDBM435735 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | The inhibition of HCMV replication by digitoxin |
|---|
| EC50 | 322±11.67 nM |
|---|
| Citation |  Boger, R Cardiac glycoside analogs and their use in methods for inhibition of viral infection US Patent US10610539 Publication Date 4/7/2020 Boger, R Cardiac glycoside analogs and their use in methods for inhibition of viral infection US Patent US10610539 Publication Date 4/7/2020 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| UL37 immediate early glycoprotein |
|---|
| Name: | UL37 immediate early glycoprotein |
|---|
| Synonyms: | UL37 | VGLI_HCMVA |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 56144.76 |
|---|
| Organism: | HHV-5 |
|---|
| Description: | P16778 |
|---|
| Residue: | 487 |
|---|
| Sequence: | MSPVYVNLLGSVGLLAFWYFSYRWIQRKRLEDPLPPWLRKKKACALTRRSRHRLRRQHGV
IDGENSETERSVDLVAALLAEAGEESVTEDTEREDTEEEREDEEEENEARTPEVNPIDAE
GLSGLAREACEALKKALRRHRFLWQRRQRARMLQHNGPQQSHHAAVFCRVHGLRGFQVSV
WLLLTLLWSTGHGVSVRCTYHGTDVNRTSNTTSMNCHLNCTRNHTQIYNGPCLGTEARLP
LNVTFNQSRRKWHSVMLKFGFQYHLEGWFPLRVLNESREINVTEVHGEVACFRNDTNVTV
GQLTLNFTGHSYVLRAIAHTSPFESYVRWEETNVTDNATSSENTTTVMSTLTKYAESDYI
FLQDMCPRFLKRTVKLTRNKTKHNVTVTGNNMTTLPVWTPECKGWTYWTTLSVMWRNRRS
ALLRAKSRALGHWALLSICTVAAGSIALLSLFCILLIGLRRDLLEDFRYICRDEGSSSTK
NDVHRIV
|
|
|
|---|
| BDBM435735 |
|---|
| n/a |
|---|
| Name | BDBM435735 |
|---|
| Synonyms: | US10610539, Compound Tris-D-rhamnose |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C61H98O24 |
|---|
| Mol. Mass. | 1215.4164 |
|---|
| SMILES | CC1OC(CC(OC2OC(C)[C@@H](OC3OC(C)[C@H](OC4OC(C)[C@H](O)C(O)[C@H]4O)C(O)[C@H]3O)C(O)[C@H]2O)C1C)OC1C(C)OC(OC2C(O)CC(O[C@H]3CC[C@@]4(C)[C@H](CC[C@@H]5[C@@H]4CC[C@]4(C)[C@H](CC[C@]54O)C4=CC(=O)OC4)C3)OC2C)C(C)C1O |r,t:78| |
|---|
| Structure | 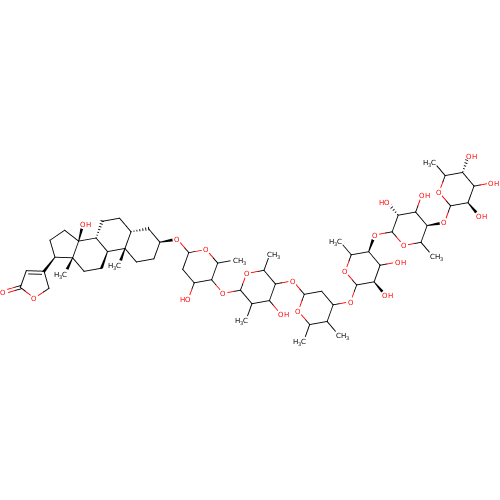
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Boger, R Cardiac glycoside analogs and their use in methods for inhibition of viral infection US Patent US10610539 Publication Date 4/7/2020
Boger, R Cardiac glycoside analogs and their use in methods for inhibition of viral infection US Patent US10610539 Publication Date 4/7/2020