| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Protein arginine N-methyltransferase 5 |
|---|
| Ligand | BDBM453028 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Biochemical Assay |
|---|
| IC50 | 2.20±n/a nM |
|---|
| Citation |  Luengo, J; Lin, H; Hawkins, M; Shetty, R; Pitis, P; Saborit Villarroya, G Selective inhibitors of protein arginine methyltransferase 5 (PRMT5) US Patent US10711007 Publication Date 7/14/2020 Luengo, J; Lin, H; Hawkins, M; Shetty, R; Pitis, P; Saborit Villarroya, G Selective inhibitors of protein arginine methyltransferase 5 (PRMT5) US Patent US10711007 Publication Date 7/14/2020 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Protein arginine N-methyltransferase 5 |
|---|
| Name: | Protein arginine N-methyltransferase 5 |
|---|
| Synonyms: | 72 kDa ICln-binding protein | ANM5_HUMAN | HRMT1L5 | Histone-arginine N-methyltransferase PRMT5 | IBP72 | JBP1 | Jak-binding protein 1 | PRMT5 | PRMT5/MEP50 complex | Protein arginine N-methyltransferase 5 (PRMT5) | Protein arginine methyltransferase 5 (PRMT5) | SKB1 | SKB1 homolog | SKB1Hs | Shk1 kinase-binding protein 1 homolog |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 72679.99 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | O14744 |
|---|
| Residue: | 637 |
|---|
| Sequence: | MAAMAVGGAGGSRVSSGRDLNCVPEIADTLGAVAKQGFDFLCMPVFHPRFKREFIQEPAK
NRPGPQTRSDLLLSGRDWNTLIVGKLSPWIRPDSKVEKIRRNSEAAMLQELNFGAYLGLP
AFLLPLNQEDNTNLARVLTNHIHTGHHSSMFWMRVPLVAPEDLRDDIIENAPTTHTEEYS
GEEKTWMWWHNFRTLCDYSKRIAVALEIGADLPSNHVIDRWLGEPIKAAILPTSIFLTNK
KGFPVLSKMHQRLIFRLLKLEVQFIITGTNHHSEKEFCSYLQYLEYLSQNRPPPNAYELF
AKGYEDYLQSPLQPLMDNLESQTYEVFEKDPIKYSQYQQAIYKCLLDRVPEEEKDTNVQV
LMVLGAGRGPLVNASLRAAKQADRRIKLYAVEKNPNAVVTLENWQFEEWGSQVTVVSSDM
REWVAPEKADIIVSELLGSFADNELSPECLDGAQHFLKDDGVSIPGEYTSFLAPISSSKL
YNEVRACREKDRDPEAQFEMPYVVRLHNFHQLSAPQPCFTFSHPNRDPMIDNNRYCTLEF
PVEVNTVLHGFAGYFETVLYQDITLSIRPETHSPGMFSWFPILFPIKQPITVREGQTICV
RFWRCSNSKKVWYEWAVTAPVCSAIHNPTGRSYTIGL
|
|
|
|---|
| BDBM453028 |
|---|
| n/a |
|---|
| Name | BDBM453028 |
|---|
| Synonyms: | (2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimidin-7- yl)-5-((S)-2-chloro-4,7- dihydro-5H-thieno[2,3- c]pyran-7-yl)tetrahydrofuran- 3,4-diol | US10711007, Example 90 | US11214574, Ex# 90 | US11254683, Example 90 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H17ClN4O4S |
|---|
| Mol. Mass. | 408.859 |
|---|
| SMILES | Nc1ncnc2n(ccc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)[C@@H]1OCCc2cc(Cl)sc12 |r| |
|---|
| Structure | 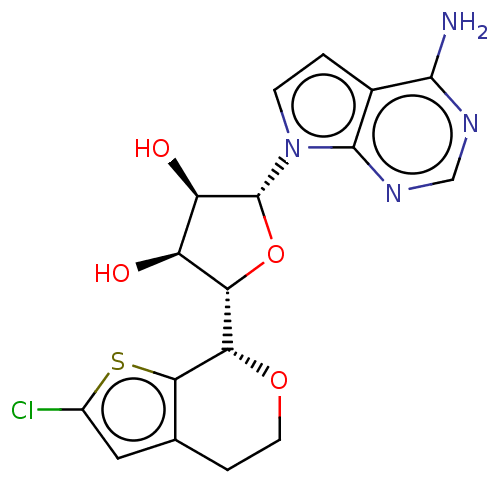
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Luengo, J; Lin, H; Hawkins, M; Shetty, R; Pitis, P; Saborit Villarroya, G Selective inhibitors of protein arginine methyltransferase 5 (PRMT5) US Patent US10711007 Publication Date 7/14/2020
Luengo, J; Lin, H; Hawkins, M; Shetty, R; Pitis, P; Saborit Villarroya, G Selective inhibitors of protein arginine methyltransferase 5 (PRMT5) US Patent US10711007 Publication Date 7/14/2020