null
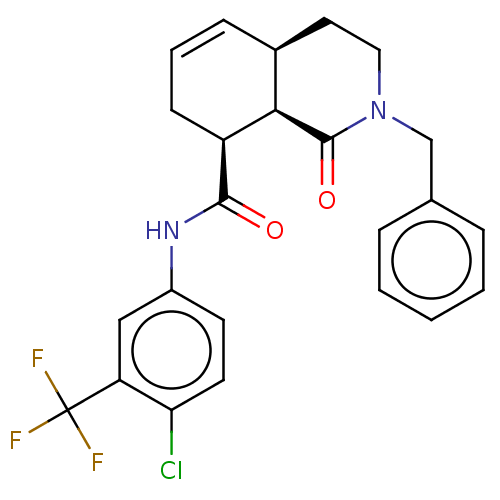
SMILES FC(F)(F)c1cc(NC(=O)[C@H]2CC=C[C@H]3CCN(Cc4ccccc4)C(=O)[C@@H]23)ccc1Cl
InChI Key InChIKey=BSPPZJZWKVYWNH-DJPFJPOOSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 214799
Found 2 hits for monomerid = 214799
TargetKappa-type opioid receptor(Homo sapiens (Human))
Departments of Molecular Medicine and Neuroscience, The Scripps Research Institute, Jupiter, Florida 33458, United States.
Curated by ChEMBL
Departments of Molecular Medicine and Neuroscience, The Scripps Research Institute, Jupiter, Florida 33458, United States.
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Agonist activity at human kappa opioid receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
Departments of Molecular Medicine and Neuroscience, The Scripps Research Institute, Jupiter, Florida 33458, United States.
Curated by ChEMBL
Departments of Molecular Medicine and Neuroscience, The Scripps Research Institute, Jupiter, Florida 33458, United States.
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as beta-arrestin2 recruitment by enzyme fragment complementation meth...More data for this Ligand-Target Pair