null
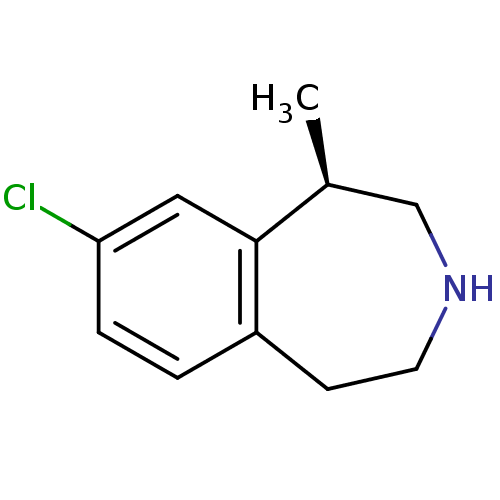
SMILES C[C@H]1CNCCc2ccc(Cl)cc12
InChI Key InChIKey=XTTZERNUQAFMOF-QMMMGPOBSA-N
PDB links: 3 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50161646
Found 3 hits for monomerid = 50161646
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Arena Pharmaceuticals Inc.
Curated by ChEMBL
Arena Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataEC50: 158nMAssay Description:Agonist activity at human 5HT2A receptor expressed in HEK293 cells assessed as intracellular IP3 accumulationMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2B(Homo sapiens (Human))
Arena Pharmaceuticals Inc.
Curated by ChEMBL
Arena Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataEC50: 794nMAssay Description:Agonist activity at human 5HT2B receptor expressed in HEK293 cells assessed as intracellular IP3 accumulationMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Arena Pharmaceuticals Inc.
Curated by ChEMBL
Arena Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataEC50: 7.90nMAssay Description:Agonist activity at human 5HT2C receptor expressed in HEK293 cells assessed as intracellular IP3 accumulationMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)