null
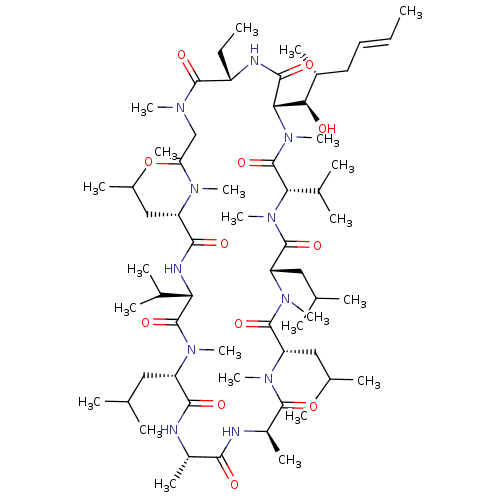
SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C
InChI Key InChIKey=PMATZTZNYRCHOR-CGLBZJNRSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50022815
Found 3 hits for monomerid = 50022815
TargetPeptidyl-prolyl cis-trans isomerase A(Homo sapiens (Human))
Sandoz Pharma Ltd.
Curated by ChEMBL
Sandoz Pharma Ltd.
Curated by ChEMBL
Affinity DataIC50: 62nMAssay Description:In vitro binding affinity of the compound against cyclophilin A by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The compound was evaluated in vitro for the immunosuppressive activity in interleukin-2 by interleukin-2 reporter gene assay (IL2-RGA)More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase A(Homo sapiens (Human))
Sandoz Pharma Ltd.
Curated by ChEMBL
Sandoz Pharma Ltd.
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:In vitro binding affinity of the compound against cyclophilin A by rotamase assayMore data for this Ligand-Target Pair