null
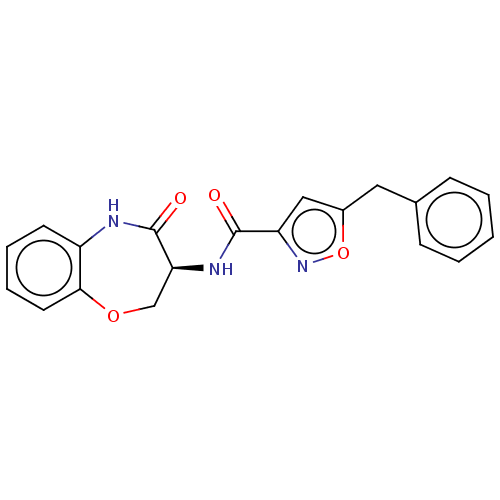
SMILES O=C(N[C@H]1COc2ccccc2NC1=O)c1cc(Cc2ccccc2)on1
InChI Key InChIKey=HMBAEAQKWTXBHE-KRWDZBQOSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50159512
Found 2 hits for monomerid = 50159512
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of pDEST8HisGSTTev-tagged human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated with enzyme for 1 hr ...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of RIP1 in human U937 cells assessed as reduction in necroptosis incubated for 24 hrs by cell titer-glo luminescent cell viability assayMore data for this Ligand-Target Pair