null
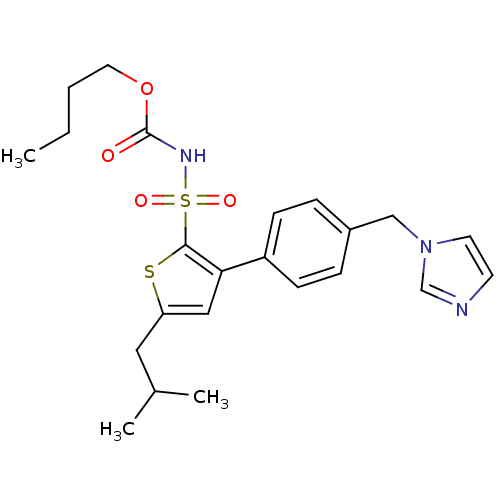
SMILES CCCCOC(=O)NS(=O)(=O)c1sc(CC(C)C)cc1-c1ccc(Cn2ccnc2)cc1
InChI Key InChIKey=XTEOJPUYZWEXFI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50156173
Found 3 hits for monomerid = 50156173
TargetType-2 angiotensin II receptor(Homo sapiens (Human))
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Binding affinity to AT2 receptor (unknown origin)More data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor A(RAT)
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]Ang II from AT1 receptor in rat liver membranesMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor(Homo sapiens (Human))
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Binding affinity to AT1 receptor (unknown origin)More data for this Ligand-Target Pair