null
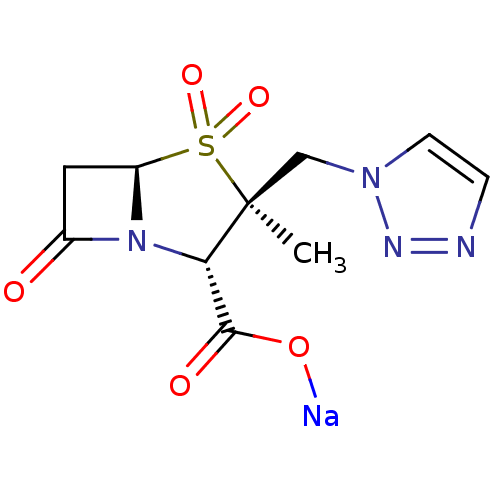
SMILES C[C@]1(Cn2ccnn2)[C@@H](N2[C@@H](CC2=O)S1(=O)=O)C(=O)O[Na]
InChI Key InChIKey=RFMIKMMOLPNEDG-QVUDESDKSA-M
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50157692
Found 3 hits for monomerid = 50157692
Affinity DataIC50: 290nMAssay Description:The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureusMore data for this Ligand-Target Pair
Affinity DataIC50: 5.19E+4nMAssay Description:Inhibition of Class C beta-lactamase from E. cloacae strain P99More data for this Ligand-Target Pair
Affinity DataIC50: 2.57E+3nMAssay Description:The compound was evaluated for inhibition against Beta-lactamase derived from Staphylococcus aureusMore data for this Ligand-Target Pair